Interplay of extracellular matrix and leukocytes in lung inflammation
- PMID: 28077237
- PMCID: PMC5290208
- DOI: 10.1016/j.cellimm.2016.12.003
Interplay of extracellular matrix and leukocytes in lung inflammation
Abstract
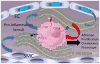
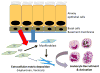
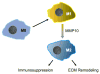
During inflammation, leukocytes influx into lung compartments and interact with extracellular matrix (ECM). Two ECM components, versican and hyaluronan, increase in a range of lung diseases. The interaction of leukocytes with these ECM components controls leukocyte retention and accumulation, proliferation, migration, differentiation, and activation as part of the inflammatory phase of lung disease. In addition, bronchial epithelial cells from asthmatic children co-cultured with human lung fibroblasts generate an ECM that is adherent for monocytes/macrophages. Macrophages are present in both early and late lung inflammation. Matrix metalloproteinase 10 (MMP10) is induced in alveolar macrophages with injury and infection and modulates macrophage phenotype and their ability to degrade collagenous ECM components. Collectively, studies outlined in this review highlight the importance of specific ECM components in the regulation of inflammatory events in lung disease. The widespread involvement of these ECM components in the pathogenesis of lung inflammation make them attractive candidates for therapeutic intervention.
Keywords: Asthma; Extracellular matrix; Fibrosis; Hyaluronan; Immunity; Inflammation; Macrophage; Matrix metalloproteinase 10; Versican.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–76. - PMC - PubMed
-
- Weibel ER, Crystal RG. Structural organization of the pulmonary interstitium. In: Crystal RG, West JB, editors. The Lung: Scientific Foundations. Raven Press; New York: 1991. pp. 369–380.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

