Serine/Threonine Phosphatases in Atrial Fibrillation
- PMID: 28077320
- PMCID: PMC5346472
- DOI: 10.1016/j.yjmcc.2016.12.009
Serine/Threonine Phosphatases in Atrial Fibrillation
Abstract
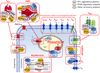
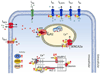
Serine/threonine protein phosphatases control dephosphorylation of numerous cardiac proteins, including a variety of ion channels and calcium-handling proteins, thereby providing precise post-translational regulation of cardiac electrophysiology and function. Accordingly, dysfunction of this regulation can contribute to the initiation, maintenance and progression of cardiac arrhythmias. Atrial fibrillation (AF) is the most common heart rhythm disorder and is characterized by electrical, autonomic, calcium-handling, contractile, and structural remodeling, which include, among other things, changes in the phosphorylation status of a wide range of proteins. Here, we review AF-associated alterations in the phosphorylation of atrial ion channels, calcium-handling and contractile proteins, and their role in AF-pathophysiology. We highlight the mechanisms controlling the phosphorylation of these proteins and focus on the role of altered dephosphorylation via local type-1, type-2A and type-2B phosphatases (PP1, PP2A, and PP2B, also known as calcineurin, respectively). Finally, we discuss the challenges for phosphatase research, potential therapeutic significance of altered phosphatase-mediated protein dephosphorylation in AF, as well as future directions.
Keywords: atrial fibrillation; calcium handling; ion channels; myofilaments; protein phosphatases.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
X.H.T.W. is a founding partner of Elex Biotech, a start-up company that developed drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders. D.D. is consultant for OMEICOS Therapeutics that develops drug molecules targeting the ω-fatty acid metabolism as an antiarrhythmic therapeutic strategy. J.H. and S.G declared no conflicts of interest.
Figures

References
-
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. - PubMed
-
- Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. - PubMed
-
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

