HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology
- PMID: 28077399
- PMCID: PMC6272805
- DOI: 10.1093/ajcp/aqw206
HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology
Abstract
Context: ERBB2 (erb-b2 receptor tyrosine kinase 2 or HER2) is currently the only biomarker established for selection of a specific therapy for patients with advanced gastroesophageal adenocarcinoma (GEA). However, there are no comprehensive guidelines for the assessment of HER2 in patients with GEA.
Objectives: To establish an evidence-based guideline for HER2 testing in patients with GEA, to formalize the algorithms for methods to improve the accuracy of HER2 testing while addressing which patients and tumor specimens are appropriate, and to provide guidance on clinical decision making.
Design: The College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology convened an expert panel to conduct a systematic review of the literature to develop an evidence-based guideline with recommendations for optimal HER2 testing in patients with GEA.
Results: The panel is proposing 11 recommendations with strong agreement from the open-comment participants.
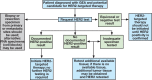
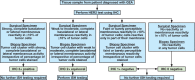
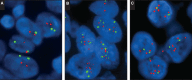
Recommendations: The panel recommends that tumor specimen(s) from all patients with advanced GEA, who are candidates for HER2-targeted therapy, should be assessed for HER2 status before the initiation of HER2-targeted therapy. Clinicians should offer combination chemotherapy and a HER2-targeted agent as initial therapy for all patients with HER2-positive advanced GEA. For pathologists, guidance is provided for morphologic selection of neoplastic tissue, testing algorithms, scoring methods, interpretation and reporting of results, and laboratory quality assurance.
Conclusions: This guideline provides specific recommendations for assessment of HER2 in patients with advanced GEA while addressing pertinent technical issues and clinical implications of the results.
Keywords: Gastroesophageal adenocarcinoma; Guidelines; HER2 testing.
© 2016 College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- National Cancer Institute. Cancer types. http://www.cancer.gov/types. Accessed July 8, 2016.
-
- World Cancer Research Fund International. Cancer facts & figures: worldwide data. http://www.wcrf.org/int/cancer-facts-figures/worldwide-data. Accessed June 28, 2016.
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. - PubMed
-
- Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644-1646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

