Protein Interactions during the Flavivirus and Hepacivirus Life Cycle
- PMID: 28077444
- PMCID: PMC5393392
- DOI: 10.1074/mcp.R116.065649
Protein Interactions during the Flavivirus and Hepacivirus Life Cycle
Abstract
Protein-protein interactions govern biological functions in cells, in the extracellular milieu, and at the border between cells and extracellular space. Viruses are small intracellular parasites and thus rely on protein interactions to produce progeny inside host cells and to spread from cell to cell. Usage of host proteins by viruses can have severe consequences e.g. apoptosis, metabolic disequilibria, or altered cell proliferation and mobility. Understanding protein interactions during virus infection can thus educate us on viral infection and pathogenesis mechanisms. Moreover, it has led to important clinical translations, including the development of new therapeutic and vaccination strategies. Here, we will discuss protein interactions of members of the Flaviviridae family, which are small enveloped RNA viruses. Dengue virus, Zika virus and hepatitis C virus belong to the most prominent human pathogenic Flaviviridae With a genome of roughly ten kilobases encoding only ten viral proteins, Flaviviridae display intricate mechanisms to engage the host cell machinery for their purpose. In this review, we will highlight how dengue virus, hepatitis C virus, Japanese encephalitis virus, tick-borne encephalitis virus, West Nile virus, yellow fever virus, and Zika virus proteins engage host proteins and how this knowledge helps elucidate Flaviviridae infection. We will specifically address the protein composition of the virus particle as well as the protein interactions during virus entry, replication, particle assembly, and release from the host cell. Finally, we will give a perspective on future challenges in Flaviviridae interaction proteomics and why we believe these challenges should be met.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Authors declare no conflict of interest
Figures
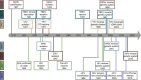
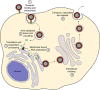
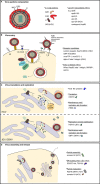
References
-
- Valentine G, Marquez L., and Pammi M. (2016) Zika virus epidemic: An update. Expert Rev. Anti Infect. Ther. 14, 1127–1138 - PubMed
-
- Monath T. P. (2008) Treatment of yellow fever. Antiviral Res. 78, 116–124 - PubMed
-
- Halstead S. B., and Thomas S. J. (2010) Japanese encephalitis: New options for active immunization. Clin. Infect. Dis. 50, 1155–1164 - PubMed
-
- Gubler D. J. (2002) The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 33, 330–342 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

