Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus
- PMID: 28077655
- PMCID: PMC5355594
- DOI: 10.1128/JVI.02079-16
Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus
Abstract
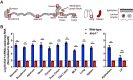
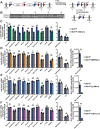
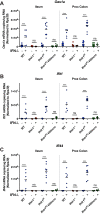
Lambda interferon (IFN-λ) has potent antiviral effects against multiple enteric viral pathogens, including norovirus and rotavirus, in both preventing and curing infection. Because the intestine includes a diverse array of cell types, however, the cell(s) upon which IFN-λ acts to exert its antiviral effects is unclear. Here, we sought to identify IFN-λ-responsive cells by generation of mice with lineage-specific deletion of the receptor for IFN-λ, Ifnlr1 We found that expression of IFNLR1 on intestinal epithelial cells (IECs) in the small intestine and colon is required for enteric IFN-λ antiviral activity. IEC Ifnlr1 expression also determines the efficacy of IFN-λ in resolving persistent murine norovirus (MNoV) infection and regulates fecal shedding and viral titers in tissue. Thus, the expression of Ifnlr1 by IECs is necessary for the response to both endogenous and exogenous IFN-λ. We further demonstrate that IEC Ifnlr1 expression is required for the sterilizing innate immune effects of IFN-λ by extending these findings in Rag1-deficient mice. Finally, we assessed whether our findings pertained to multiple viral pathogens by infecting mice specifically lacking IEC Ifnlr1 expression with reovirus. These mice phenocopied Ifnlr1-null animals, exhibiting increased intestinal tissue titers and enhanced reovirus fecal shedding. Thus, IECs are the critical cell type responding to IFN-λ to control multiple enteric viruses. This is the first genetic evidence that supports an essential role for IECs in IFN-λ-mediated control of enteric viral infection, and these findings provide insight into the mechanism of IFN-λ-mediated antiviral activity.IMPORTANCE Human noroviruses (HNoVs) are the leading cause of epidemic gastroenteritis worldwide. Type III interferons (IFN-λ) control enteric viral infections in the gut and have been shown to cure mouse norovirus, a small-animal model for HNoVs. Using a genetic approach with conditional knockout mice, we identified IECs as the dominant IFN-λ-responsive cells in control of enteric virus infection in vivo Upon murine norovirus or reovirus infection, Ifnlr1 depletion in IECs largely recapitulated the phenotype seen in Ifnlr1-/- mice of higher intestinal tissue viral titers and increased viral shedding in the stool. Moreover, IFN-λ-mediated sterilizing immunity against murine norovirus requires the capacity of IECs to respond to IFN-λ. These findings clarify the mechanism of action of this cytokine and emphasize the therapeutic potential of IFN-λ for treating mucosal viral infections.
Keywords: innate immunity; interferons; mucosal immunity; norovirus; reovirus.
Copyright © 2017 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

