Connexin-Mediated Signaling in Nonsensory Cells Is Crucial for the Development of Sensory Inner Hair Cells in the Mouse Cochlea
- PMID: 28077706
- PMCID: PMC5242392
- DOI: 10.1523/JNEUROSCI.2251-16.2016
Connexin-Mediated Signaling in Nonsensory Cells Is Crucial for the Development of Sensory Inner Hair Cells in the Mouse Cochlea
Abstract
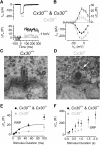
Mutations in the genes encoding for gap junction proteins connexin 26 (Cx26) and connexin 30 (Cx30) have been linked to syndromic and nonsyndromic hearing loss in mice and humans. The release of ATP from connexin hemichannels in cochlear nonsensory cells has been proposed to be the main trigger for action potential activity in immature sensory inner hair cells (IHCs), which is crucial for the refinement of the developing auditory circuitry. Using connexin knock-out mice, we show that IHCs fire spontaneous action potentials even in the absence of ATP-dependent intercellular Ca2+ signaling in the nonsensory cells. However, this signaling from nonsensory cells was able to increase the intrinsic IHC firing frequency. We also found that connexin expression is key to IHC functional maturation. In Cx26 conditional knock-out mice (Cx26Sox10-Cre), the maturation of IHCs, which normally occurs at approximately postnatal day 12, was partially prevented. Although Cx30 has been shown not to be required for hearing in young adult mice, IHCs from Cx30 knock-out mice exhibited a comprehensive brake in their development, such that their basolateral membrane currents and synaptic machinery retain a prehearing phenotype. We propose that IHC functional differentiation into mature sensory receptors is initiated in the prehearing cochlea provided that the expression of either connexin reaches a threshold level. As such, connexins regulate one of the most crucial functional refinements in the mammalian cochlea, the disruption of which contributes to the deafness phenotype observed in mice and DFNB1 patients.
Significance statement: The correct development and function of the mammalian cochlea relies not only on the sensory hair cells, but also on the surrounding nonsensory cells. Although the nonsensory cells have been largely implicated in the general homeostasis in the mature cochlea, their involvement in the initial functional differentiation of the sensory inner hair cells is less clear. Using mutant mouse models for the most common form of congenital deafness in humans, which are knock-outs for the gap-junction channels connexin 26 and connexin 30 genes, we show that defects in nonsensory cells prevented the functional maturation of inner hair cells. In connexin knock-outs, inner hair cells remained stuck at a prehearing stage of development and, as such, are unable to process sound information.
Keywords: cochlea; connexin; deafness; development; gap-junction; inner hair cells.
Copyright © 2017 Johnson, Ceriani et al.
Figures




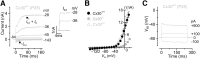

References
-
- Ahmad S, Tang W, Chang Q, Qu Y, Hibshman J, Li Y, Söhl G, Willecke K, Chen P, Lin X. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci U S A. 2007;104:1337–1341. doi: 10.1073/pnas.0606855104. - DOI - PMC - PubMed
-
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
