Excitatory Hindbrain-Forebrain Communication Is Required for Cisplatin-Induced Anorexia and Weight Loss
- PMID: 28077715
- PMCID: PMC5242394
- DOI: 10.1523/JNEUROSCI.2714-16.2016
Excitatory Hindbrain-Forebrain Communication Is Required for Cisplatin-Induced Anorexia and Weight Loss
Abstract
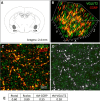
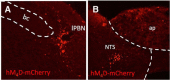
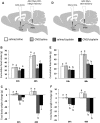
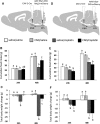
Cisplatin chemotherapy is commonly used to treat cancer despite severe energy balance side effects. In rats, cisplatin activates nucleus tractus solitarius (NTS) projections to the lateral parabrachial nucleus (lPBN) and calcitonin-gene related peptide (CGRP) projections from the lPBN to the central nucleus of the amygdala (CeA). We demonstrated previously that CeA glutamate receptor signaling mediates cisplatin-induced anorexia and body weight loss. Here, we used neuroanatomical tracing, immunofluorescence, and confocal imaging to demonstrate that virtually all NTS→lPBN and lPBN→CeA CGRP projections coexpress vesicular glutamate transporter 2 (VGLUT2), providing evidence that excitatory projections mediate cisplatin-induced energy balance dysregulation. To test whether lPBN→CeA projection neurons are required for cisplatin-induced anorexia and weight loss, we inhibited these neurons chemogenetically using a retrograde Cre-recombinase-expressing canine adenovirus-2 in combination with Cre-dependent inhibitory Designer Receptors Exclusive Activated by Designer Drugs (DREADDs) before cisplatin treatment. Inhibition of lPBN→CeA neurons attenuated cisplatin-induced anorexia and body weight loss significantly. Using a similar approach, we additionally demonstrated that inhibition of NTS→lPBN neurons attenuated cisplatin-induced anorexia and body weight loss significantly. Together, our data support the view that excitatory hindbrain-forebrain projections are necessary for cisplatin's untoward effects on energy intake, elucidating a key neuroanatomical circuit driving pathological anorexia and weight loss that accompanies chemotherapy treatment.
Significance statement: Chemotherapy treatments are commonly used to treat cancers despite accompanying anorexia and weight loss that may limit treatment adherence and reduce patient quality of life. Strikingly, we lack a neural understanding of, and effective treatments for, chemotherapy-induced anorexia and weight loss. The current data characterize the excitatory nature of neural projections activated by cisplatin in rats and reveal the necessity of specific hindbrain-forebrain projections for cisplatin-induced anorexia and weight loss. Together, these findings help to characterize the neural mechanisms mediating cisplatin-induced anorexia, advancing opportunities to develop better-tolerated chemotherapies and adjuvant therapies to prevent anorexia and concurrent nutritional deficiencies during cancer treatment.
Keywords: amygdala; anorexia; chemotherapy; cisplatin; glutamate; hindbrain.
Copyright © 2017 the authors 0270-6474/17/370362-09$15.00/0.
Figures

References
-
- Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate receptors in the central nucleus of the amygdala mediate cisplatin-induced malaise and energy balance dysregulation through direct hindbrain projections. J Neurosci. 2015;35:11094–11104. doi: 10.1523/JNEUROSCI.0440-15.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
