POLG2 deficiency causes adult-onset syndromic sensory neuropathy, ataxia and parkinsonism
- PMID: 28078310
- PMCID: PMC5221457
- DOI: 10.1002/acn3.361
POLG2 deficiency causes adult-onset syndromic sensory neuropathy, ataxia and parkinsonism
Abstract
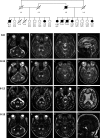
Objective: Mitochondrial dysfunction plays a key role in the pathophysiology of neurodegenerative disorders such as ataxia and Parkinson's disease. We describe an extended Belgian pedigree where seven individuals presented with adult-onset cerebellar ataxia, axonal peripheral ataxic neuropathy, and tremor, in variable combination with parkinsonism, seizures, cognitive decline, and ophthalmoplegia. We sought to identify the underlying molecular etiology and characterize the mitochondrial pathophysiology of this neurological syndrome.
Methods: Clinical, neurophysiological, and neuroradiological evaluations were conducted. Patient muscle and cultured fibroblasts underwent extensive analyses to assess mitochondrial function. Genetic studies including genome-wide sequencing were conducted.
Results: Hallmarks of mitochondrial dysfunction were present in patients' tissues including ultrastructural anomalies of mitochondria, mosaic cytochrome c oxidase deficiency, and multiple mtDNA deletions. We identified a splice acceptor variant in POLG2, c.970-1G>C, segregating with disease in this family and associated with a concomitant decrease in levels of POLG2 protein in patient cells.
Interpretation: This work extends the clinical spectrum of POLG2 deficiency to include an overwhelming, adult-onset neurological syndrome that includes cerebellar syndrome, peripheral neuropathy, tremor, and parkinsonism. We therefore suggest to include POLG2 sequencing in the evaluation of ataxia and sensory neuropathy in adults, especially when it is accompanied by tremor or parkinsonism with white matter disease. The demonstration that deletions of mtDNA resulting from autosomal-dominant POLG2 variant lead to a monogenic neurodegenerative multicomponent syndrome provides further evidence for a major role of mitochondrial dysfunction in the pathomechanism of nonsyndromic forms of the component neurodegenerative disorders.
Figures


References
-
- Sommerville EW, Chinnery PF, Gorman GS, et al. Adult‐onset Mendelian PEO associated with mitochondrial disease. J Neuromusc Dis 2014;1:119–133. - PubMed
-
- Walter MC, Czermin B, Muller‐Ziermann S, et al. Late‐onset ptosis and myopathy in a patient with a heterozygous insertion in POLG2 . J Neurol 2010;257:1517–1523. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

