Melanoma in congenital melanocytic naevi
- PMID: 28078671
- PMCID: PMC5484991
- DOI: 10.1111/bjd.15301
Melanoma in congenital melanocytic naevi
Abstract
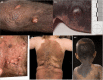
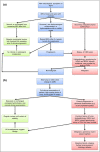
Congenital melanocytic naevi (CMN) are a known risk factor for melanoma, with the greatest risk currently thought to be in childhood. There has been controversy over the years about the incidence of melanoma, and therefore over the clinical management of CMN, due partly to the difficulties of histological diagnosis and partly to publishing bias towards cases of malignancy. Large cohort studies have demonstrated that melanoma risk in childhood is related to the severity of the congenital phenotype. New understanding of the genetics of CMN offers the possibility of improvement in diagnosis of melanoma, identification of those at highest risk, and new treatment options. We review the world literature and our centre's experience over the last 25 years, including the molecular characteristics of melanoma in these patients and new melanoma incidence and outcome data from our prospective cohort. Management strategies are proposed for presentation of suspected melanoma of the skin and the central nervous system in patients with CMN, including use of oral mitogen-activated protein kinase kinase inhibitors in NRAS-mutated tumours.
© 2017 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures


Comment in
-
Melanoma risk in congenital melanocytic naevi.Br J Dermatol. 2017 May;176(5):1114. doi: 10.1111/bjd.15477. Br J Dermatol. 2017. PMID: 28504374 No abstract available.
References
-
- Alper JC, Holmes LB. The incidence and significance of birthmarks in a cohort of 4,641 newborns. Pediatr Dermatol 1983; 1:58–68. - PubMed
-
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics 1976; 58:218–22. - PubMed
-
- Krengel S, Scope A, Dusza SW et al New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol 2013; 68:441–51. - PubMed
-
- Castilla EE, da Graca Dutra M, Orioli‐Parreiras IM. Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. Br J Dermatol 1981; 104:307–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

