C5a Increases the Injury to Primary Neurons Elicited by Fibrillar Amyloid Beta
- PMID: 28078911
- PMCID: PMC5298486
- DOI: 10.1177/1759091416687871
C5a Increases the Injury to Primary Neurons Elicited by Fibrillar Amyloid Beta
Abstract
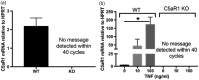
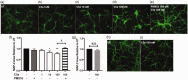
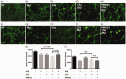
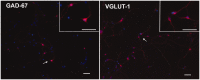
C5aR1, the proinflammatory receptor for C5a, is expressed in the central nervous system on microglia, endothelial cells, and neurons. Previous work demonstrated that the C5aR1 antagonist, PMX205, decreased amyloid pathology and suppressed cognitive deficits in two Alzheimer's Disease (AD) mouse models. However, the cellular mechanisms of this protection have not been definitively demonstrated. Here, primary cultured mouse neurons treated with exogenous C5a show reproducible loss of MAP-2 staining in a dose-dependent manner within 24 hr of treatment, indicative of injury to neurons. This injury is prevented by the C5aR1 antagonist PMX53, a close analog of PMX205. Furthermore, primary neurons derived from C5aR1 null mice exhibited no MAP-2 loss after exposure to the highest concentration of C5a tested. Primary mouse neurons treated with both 100 nM C5a and 5 µM fibrillar amyloid beta (fAβ), to model what occurs in the AD brain, showed increased MAP-2 loss relative to either C5a or fAβ alone. Blocking C5aR1 with PMX53 (100 nM) blocked the loss of MAP2 in these primary neurons to the level seen with fAβ alone. Similar experiments with primary neurons derived from C5aR1 null mice showed a loss of MAP-2 due to fAβ treatment. However, the addition of C5a to the cultures did not enhance the loss of MAP-2 and the addition of PMX53 to the cultures did not change the MAP-2 loss in response to fAβ. Thus, at least part of the beneficial effects of C5aR1 antagonist in AD mouse models may be due to protection of neurons from the toxic effects of C5a.
Keywords: C5a; C5aR1; amyloid; complement; neurodegeneration; primary neurons.
Figures
References
-
- Afagh A., Cummings B. J., Cribbs D. H., Cotman C. W., Tenner A. J. (1996) Localization and cell association of C1q in Alzheimer's disease brain. Experimental Neurology 138: 22–32. - PubMed
-
- Aizenstein H. J., Nebes R. D., Saxton J. A., Price J. C., Mathis C. A., Tsopelas N. D., Ziolko S. K., James J. A., Snitz B. E., Houck P. R., Bi W., Cohen A. D., Lopresti B. J., DeKosky S. T., Halligan E. M., Klunk W. E. (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology 65: 1509–1517. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

