Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk
- PMID: 28079186
- PMCID: PMC5228034
- DOI: 10.1038/srep40476
Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk
Abstract
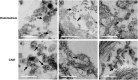
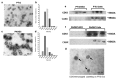
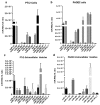
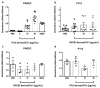
Exosomes and microvesicles are extracellular vesicles released from cells and can contain lipids, miRNAs and proteins that affect cells at distant sites. Recently, microvesicles containing miRNA have been implicated in uterine microenvironment of pigs, a species with unique epitheliochorial (non-invasive) placentation. Here we report a novel role of conceptus-derived exosomes/microvesicles (hereafter referred to as extracellular vesicles; EVs) in embryo-endometrial cross-talk. We also demonstrate the stimulatory effects of EVs (PTr2-Exo) derived from porcine trophectoderm-cells on various biological processes including the proliferation of maternal endothelial cells (PAOEC), potentially promoting angiogenesis. Transmission immuno-electron microscopy confirmed the presence of EVs in tissue biopsies, PTr2-Exo and PAOEC-derived EVs (PAOEC-Exo). RT-PCR detected 14 select miRNAs in CD63 positive EVs in which miR-126-5P, miR-296-5P, miR-16, and miR-17-5P were the most abundant angiogenic miRNAs. Proteomic analysis revealed EV proteins that play a role in angiogenesis. In-vitro experiments, using two representative cell lines of maternal-fetal interface, demonstrated bidirectional EVs shuttling between PTr2 and PAOEC cells. Importantly, these studies support the idea that PTr2-Exo and PAOEC-Exo containing select miRNAs and proteins can be successfully delivered to recipient cells and that they may have a biological role in conceptus-endometrial cross-talk crucial for the pregnancy success.
Figures




References
-
- Valadi H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007). - PubMed
-
- Simpson R. J., Jensen S. S. & Lim J. W. E. Proteomic profiling of exosomes: Current perspectives. Proteomics 8, 4083–4099 (2008). - PubMed
-
- Luo S. S. et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol. Reprod. 81, 717–729 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

