Postischemic Inflammation in Acute Stroke
- PMID: 28079313
- PMCID: PMC5242162
- DOI: 10.3988/jcn.2017.13.1.1
Postischemic Inflammation in Acute Stroke
Abstract
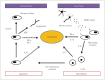
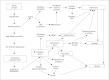
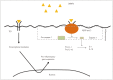
Cerebral ischemia is caused by arterial occlusion due to a thrombus or an embolus. Such occlusion induces multiple and concomitant pathophysiological processes that involve bioenergetic failure, acidosis, loss of cell homeostasis, excitotoxicity, and disruption of the blood-brain barrier. All of these mechanisms contribute to neuronal death, mainly via apoptosis or necrosis. The immune system is involved in this process in the early phases after brain injury, which contributes to potential enlargement of the infarct size and involves the penumbra area. Whereas inflammation and the immune system both exert deleterious effects, they also contribute to brain protection by stimulating a preconditioning status and to the concomitant repair of the injured parenchyma. This review describes the main phases of the inflammatory process occurring after arterial cerebral occlusion, with an emphasis on the role of single mediators.
Keywords: immune response; inflammation; ischemic stroke.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
References
-
- Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. - PubMed
-
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

