PET imaging of cannabinoid type 2 receptors with [11C]A-836339 did not evidence changes following neuroinflammation in rats
- PMID: 28079433
- PMCID: PMC5363492
- DOI: 10.1177/0271678X16685105
PET imaging of cannabinoid type 2 receptors with [11C]A-836339 did not evidence changes following neuroinflammation in rats
Abstract
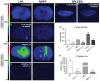
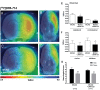
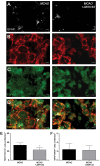
Cannabinoid type 2 receptors (CB2R) have emerged as promising targets for the diagnosis and therapy of brain pathologies. However, no suitable radiotracers for accurate CB2R mapping have been found to date, limiting the investigation of the CB2 receptor expression using positron emission tomography (PET) imaging. In this work, we report the evaluation of the in vivo expression of CB2R with [11C]A-836339 PET after cerebral ischemia and in two rat models of neuroinflammation, first by intrastriatal LPS and then by AMPA injection. PET images and in vitro autoradiography showed a lack of specific [11C]A-836339 uptake in these animal models demonstrating the limitation of this radiotracer to image CB2 receptor under neuroinflammatory conditions. Further, using immunohistochemistry, the CB2 receptor displayed a modest expression increase after cerebral ischemia, LPS and AMPA models. Finally, [18F]DPA-714-PET and immunohistochemistry demonstrated decreased neuroinflammation by a selective CB2R agonist, JWH133. Taken together, these findings suggest that [11C]A-836339 is not a suitable radiotracer to monitor in vivo CB2R expression by using PET imaging. Future studies will have to investigate alternative radiotracers that could provide an accurate binding to CB2 receptors following brain inflammation.
Keywords: TSPO; [11C]A-836339; [18F]DPA-714; cannabinoid type 2 receptors; cerebral ischemia; neuroinflammation; positron emission tomography.
Figures
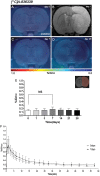
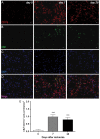
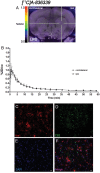
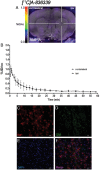
References
-
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 2005; 168: 299–325. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

