Amplification of HER2 and TOP2A and deletion of TOP2A genes in a series of Taiwanese breast cancer
- PMID: 28079792
- PMCID: PMC5266154
- DOI: 10.1097/MD.0000000000005582
Amplification of HER2 and TOP2A and deletion of TOP2A genes in a series of Taiwanese breast cancer
Abstract
Background: The prognostic relevance of topoisomerase II alpha (TOP2A) copy number change remains not well established. This study is aimed to investigate the frequency and pattern of TOP2A aberrations; to correlate TOP2A alterations with human epidermal growth factor receptor 2 (HER2) status and clinicopathological parameters, and further to explore prognostic value of TOP2A and HER2 status in breast cancer in Taiwan.
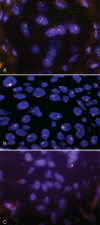
Methods: We analyzed tissue samples from 311 invasive carcinomas in tissue microarrays for TOP2A and HER2 status by fluorescent in situ hybridization.
Results: TOP2A copy number change is an infrequent genetic event (9.8% amplification and 2.7% deletion) and is present in both HER2-amplified and nonamplified tumors. TOP2A amplification is statistically associated with age >50 at diagnosis (P = 0.016) and HER2 amplification (P < 0.001). HER2 amplification, but not TOP2A amplification, is a predictor of unfavorable prognosis (P = 0.002). Univariate and multivariate analysis showed that higher histologic grading, positive nodal involvement, and HER2 positivity were associated with poorer overall survival. Cytogenetically, double minutes-type amplification is the predominant pattern for both genes (HER2: 64% and TOP2A: 93.1%). Homogeneous staining region-type signals of both genes are resistant to RNase digestion, supporting that these were not nuclear accumulation of mRNA transcripts.
Conclusion: Our results demonstrate the prognostic value of tumor grading, nodal involvement, and HER2 status in Taiwanese breast cancer. TOP2A aberrations are an infrequent event independent of HER2 status, and TOP2A amplification carries no prognostic value. The predictive value of TOP2A aberrations in patients of breast cancer taking athracycline-containing treatment in Taiwan remains to be determined in prospectively well-designed clinical trials.
Conflict of interest statement
The authors have declared that no conflicting interests exist.
Figures



References
-
- Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO Classification of Tumours of the Breast, Fourth Edition. Lyon:IARC; 2012.
-
- Arriola E, Marchio C, Tan DS, et al. Genomic analysis of the HER2/TOP2A amplicon in breast cancer and breast cancer cell lines. Lab Invest 2008;88:491–503. - PubMed
-
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

