Impaired embryonic development in glucose-6-phosphate dehydrogenase-deficient Caenorhabditis elegans due to abnormal redox homeostasis induced activation of calcium-independent phospholipase and alteration of glycerophospholipid metabolism
- PMID: 28079896
- PMCID: PMC5386372
- DOI: 10.1038/cddis.2016.463
Impaired embryonic development in glucose-6-phosphate dehydrogenase-deficient Caenorhabditis elegans due to abnormal redox homeostasis induced activation of calcium-independent phospholipase and alteration of glycerophospholipid metabolism
Abstract
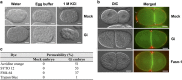
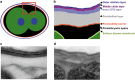
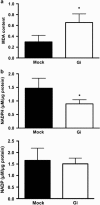
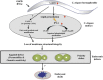
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a commonly pervasive inherited disease in many parts of the world. The complete lack of G6PD activity in a mouse model causes embryonic lethality. The G6PD-deficient Caenorhabditis elegans model also shows embryonic death as indicated by a severe hatching defect. Although increased oxidative stress has been implicated in both cases as the underlying cause, the exact mechanism has not been clearly delineated. In this study with C. elegans, membrane-associated defects, including enhanced permeability, defective polarity and cytokinesis, were found in G6PD-deficient embryos. The membrane-associated abnormalities were accompanied by impaired eggshell structure as evidenced by a transmission electron microscopic study. Such loss of membrane structural integrity was associated with abnormal lipid composition as lipidomic analysis revealed that lysoglycerophospholipids were significantly increased in G6PD-deficient embryos. Abnormal glycerophospholipid metabolism leading to defective embryonic development could be attributed to the increased activity of calcium-independent phospholipase A2 (iPLA) in G6PD-deficient embryos. This notion is further supported by the fact that the suppression of multiple iPLAs by genetic manipulation partially rescued the embryonic defects in G6PD-deficient embryos. In addition, G6PD deficiency induced disruption of redox balance as manifested by diminished NADPH and elevated lipid peroxidation in embryos. Taken together, disrupted lipid metabolism due to abnormal redox homeostasis is a major factor contributing to abnormal embryonic development in G6PD-deficient C. elegans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Luzzatto L. G6PD deficiency: a polymorphism balanced by heterozygote advantage against malaria. Lancet Haematol 2015; 2: e400–e401. - PubMed
-
- Yang HC, Wu YH, Liu HY, Stern A, Chiu DT. What has passed is prolog: new cellular and physiological roles of G6PD. Free Radic Res 2016; 50: 1047–1064. - PubMed
-
- Mazieres S, Petit F, Dugoujon JM, Iriart X, Berry A, Carme B et al. Subtle adjustments of the glucose-6-phosphate dehydrogenase (G6PD) mutation database and reference sequence. Blood Cells Mol Dis 2014; 52: 55–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

