Maresin 1 induces a novel pro-resolving phenotype in human platelets
- PMID: 28079976
- PMCID: PMC5378657
- DOI: 10.1111/jth.13620
Maresin 1 induces a novel pro-resolving phenotype in human platelets
Abstract
Essentials Specialized proresolving mediators (SPMs) promote the resolution of inflammation. This study sought to investigate the effects of SPMs on human platelet function. The SPM, Maresin 1, enhanced hemostatic, but suppressed inflammatory functions of platelets. SPMs uniquely regulate platelet function and may represent a new class of antiplatelet agents.
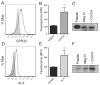
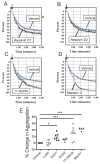
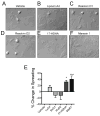
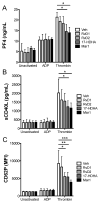
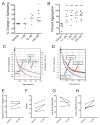
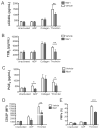
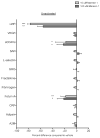
Summary: Background Antiplatelet therapy is a cornerstone of modern medical practice and is routinely employed to reduce the likelihood of myocardial infarction, thrombosis and stroke. However, current antiplatelet therapies, such as aspirin, often have adverse side-effects, including increased risk of bleeding, and some patients are relatively 'aspirin-resistant'. Platelets are intimately involved in hemostasis and inflammation, and clinical consequences are associated with excessive or insufficient platelet activation. Objectives A major unmet need in the field of hematology is the development of new agents that safely prevent unwanted platelet activation in patients with underlying cardiovascular disease, while minimizing the risk of bleeding. Here, we investigate the potential of endogenously produced, specialized pro-resolving mediators (SPMs) as novel antiplatelet agents. SPMs are a recently discovered class of lipid-derived molecules that drive the resolution of inflammation without being overtly immunosuppressive. Methods Human platelets were treated with lipoxin A4, resolvin D1, resolvin D2, 17-HDHA or maresin 1 for 15 min, then were subjected to platelet function tests, including spreading, aggregation and inflammatory mediator release. Results We show for the first time that human platelets express the SPM receptors, GPR32 and ALX. Furthermore, our data demonstrate that maresin 1 differentially regulates platelet hemostatic function by enhancing platelet aggregation and spreading, while suppressing release of proinflammatory and prothrombotic mediators. Conclusions These data support the concept that SPMs differentially regulate platelet function and may represent a novel class of antiplatelet agents. SPMs also may play an important role in the resolution of inflammation in cardiovascular diseases.
Keywords: antiplatelet agents; hemostasis; inflammation; platelet activation; platelets.
© 2017 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
N. Blumberg has received lecture honoraria and consulting fees from Antek, Inc., Fenwal, Pall BioMedical, and Caridian (Terumo), manufacturers of leukoreduction filters, blood component equipment, and cell washing devices. The other authors have nothing to disclose.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

