Is the cardiac monitoring function related to the self in both the default network and right anterior insula?
- PMID: 28080963
- PMCID: PMC5062094
- DOI: 10.1098/rstb.2016.0004
Is the cardiac monitoring function related to the self in both the default network and right anterior insula?
Abstract
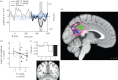
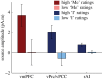
The self has been proposed to be rooted in the neural monitoring of internal bodily signals and might thus involve interoceptive areas, notably the right anterior insula (rAI). However, studies on the self consistently showed the involvement of midline default network (DN) nodes, without referring to visceral monitoring. Here, we investigate this apparent discrepancy. We previously showed that neural responses to heartbeats in the DN encode two different self-dimensions, the agentive 'I' and the introspective 'Me', in a whole-brain analysis of magnetoencephalography (MEG) data. Here, we confirm and anatomically refine this result with intracranial recordings (intracranial electroencephalography, iEEG). In two patients, we show a parametric modulation of neural responses to heartbeats by the self-relatedness of thoughts, at the single trial level. A region-of-interest analysis of the insula reveals that MEG responses to heartbeats in the rAI encode the 'I' self-dimension. The effect in rAI was weaker than in the DN and was replicated in iEEG data in one patient out of two. We propose that a common mechanism, the neural monitoring of cardiac signals, underlies the self in both the DN and rAI. This might reconcile studies on the self highlighting the DN, with studies on interoception focusing on the insula.This article is part of the themed issue 'Interoception beyond homeostasis: affect, cognition and mental health'.
Keywords: heartbeat-evoked responses; interoception; intracranial electroencephalography; magnetoencephalography; neural responses to heartbeats; spontaneous cognition.
© 2016 The Authors.
Figures




References
-
- Damasio AR. 1999. The feeling of what happens: body, emotion and the making of consciousness. New York, NY: Harcourt.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
