An active inference theory of allostasis and interoception in depression
- PMID: 28080969
- PMCID: PMC5062100
- DOI: 10.1098/rstb.2016.0011
An active inference theory of allostasis and interoception in depression
Abstract
In this paper, we integrate recent theoretical and empirical developments in predictive coding and active inference accounts of interoception (including the Embodied Predictive Interoception Coding model) with working hypotheses from the theory of constructed emotion to propose a biologically plausible unified theory of the mind that places metabolism and energy regulation (i.e. allostasis), as well as the sensory consequences of that regulation (i.e. interoception), at its core. We then consider the implications of this approach for understanding depression. We speculate that depression is a disorder of allostasis, whose myriad symptoms result from a 'locked in' brain that is relatively insensitive to its sensory context. We conclude with a brief discussion of the ways our approach might reveal new insights for the treatment of depression.This article is part of the themed issue 'Interoception beyond homeostasis: affect, cognition and mental health'.
Keywords: fMRI; interoception; major depressive disorder; prediction; visceromotor.
© 2016 The Author(s).
Figures
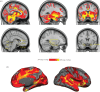

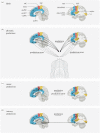
References
-
- Sterling P, Laughlin SB. 2015. Principles of neural design. Cambridge, MA: MIT Press.
-
- Deneve S, Jardri R. 2016. Circular inference: mistaken belief, misplaced trust. Curr. Opin. Behav. Sci. 11, 40–48. ( 10.1016/j.cobeha.2016.04.001) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
