Drivers of variation in species impacts for a multi-host fungal disease of bats
- PMID: 28080982
- PMCID: PMC5095535
- DOI: 10.1098/rstb.2015.0456
Drivers of variation in species impacts for a multi-host fungal disease of bats
Abstract
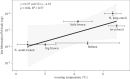
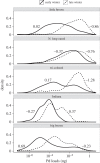
Disease can play an important role in structuring species communities because the effects of disease vary among hosts; some species are driven towards extinction, while others suffer relatively little impact. Why disease impacts vary among host species remains poorly understood for most multi-host pathogens, and factors allowing less-susceptible species to persist could be useful in conserving highly affected species. White-nose syndrome (WNS), an emerging fungal disease of bats, has decimated some species while sympatric and closely related species have experienced little effect. We analysed data on infection prevalence, fungal loads and environmental factors to determine how variation in infection among sympatric host species influenced the severity of WNS population impacts. Intense transmission resulted in almost uniformly high prevalence in all species. By contrast, fungal loads varied over 3 orders of magnitude among species, and explained 98% of the variation among species in disease impacts. Fungal loads increased with hibernating roosting temperatures, with bats roosting at warmer temperatures having higher fungal loads and suffering greater WNS impacts. We also found evidence of a threshold fungal load, above which the probability of mortality may increase sharply, and this threshold was similar for multiple species. This study demonstrates how differences in behavioural traits among species-in this case microclimate preferences-that may have been previously adaptive can be deleterious after the introduction of a new pathogen. Management to reduce pathogen loads rather than exposure may be an effective way of reducing disease impact and preventing species extinctions.This article is part of the themed issue 'Tackling emerging fungal threats to animal health, food security and ecosystem resilience'.
Keywords: Geomyces destructans; Myotis lucifugus; emerging infectious disease; multi-host pathogen; white-nose syndrome; wildlife disease.
© 2016 The Author(s).
Figures



Similar articles
-
Host persistence or extinction from emerging infectious disease: insights from white-nose syndrome in endemic and invading regions.Proc Biol Sci. 2016 Mar 16;283(1826):20152861. doi: 10.1098/rspb.2015.2861. Proc Biol Sci. 2016. PMID: 26962138 Free PMC article.
-
Pseudogymnoascus destructans transcriptome changes during white-nose syndrome infections.Virulence. 2017 Nov 17;8(8):1695-1707. doi: 10.1080/21505594.2017.1342910. Epub 2017 Jul 13. Virulence. 2017. PMID: 28614673 Free PMC article.
-
Effects of white-nose syndrome on regional population patterns of 3 hibernating bat species.Conserv Biol. 2016 Oct;30(5):1048-59. doi: 10.1111/cobi.12690. Epub 2016 Jul 18. Conserv Biol. 2016. PMID: 26872411
-
Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats.Conserv Biol. 2011 Apr;25(2):223-31. doi: 10.1111/j.1523-1739.2010.01638.x. Epub 2011 Feb 1. Conserv Biol. 2011. PMID: 21284732 Review.
-
A Palearctic view of a bat fungal disease.Conserv Biol. 2025 Feb;39(1):e14265. doi: 10.1111/cobi.14265. Epub 2024 Apr 15. Conserv Biol. 2025. PMID: 38616727 Free PMC article. Review.
Cited by
-
Host-pathogen interactions under pressure: A review and meta-analysis of stress-mediated effects on disease dynamics.Ecol Lett. 2023 Nov;26(11):2003-2020. doi: 10.1111/ele.14319. Epub 2023 Oct 7. Ecol Lett. 2023. PMID: 37804128 Free PMC article. Review.
-
Towards a mechanistic understanding of competence: a missing link in diversity-disease research.Parasitology. 2020 Sep;147(11):1159-1170. doi: 10.1017/S0031182020000943. Epub 2020 Jun 10. Parasitology. 2020. PMID: 32517830 Free PMC article.
-
Thermally unstable roosts influence winter torpor patterns in a threatened bat species.Conserv Physiol. 2024 Apr 16;12(1):coae014. doi: 10.1093/conphys/coae014. eCollection 2024. Conserv Physiol. 2024. PMID: 40242594 Free PMC article.
-
Tackling emerging fungal threats to animal health, food security and ecosystem resilience.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 5;371(1709):20160332. doi: 10.1098/rstb.2016.0332. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 28080997 Free PMC article.
-
Personality affects dynamics of an experimental pathogen in little brown bats.R Soc Open Sci. 2020 Sep 16;7(9):200770. doi: 10.1098/rsos.200770. eCollection 2020 Sep. R Soc Open Sci. 2020. PMID: 33047038 Free PMC article.
References
-
- Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4, 125–134. (10.1007/s10393-007-0093-5) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

