Ploidy dynamics and evolvability in fungi
- PMID: 28080987
- PMCID: PMC5095540
- DOI: 10.1098/rstb.2015.0461
Ploidy dynamics and evolvability in fungi
Abstract
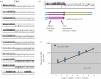
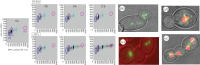
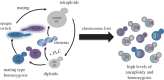
Rapid responses to acute stresses are essential for stress survival and are critical to the ability of fungal pathogens to adapt to new environments or hosts. The rapid emergence of drug resistance is used as a model for how fungi adapt and survive stress conditions that inhibit the growth of progenitor cells. Aneuploidy and loss of heterozygosity (LOH), which are large-scale genome shifts involving whole chromosomes or chromosome arms, occur at higher frequency than point mutations and have the potential to mediate stress survival. Furthermore, the stress of exposure to an antifungal drug can induce elevated levels of LOH and can promote the formation of aneuploids. This occurs via mitotic defects that first produce tetraploid progeny with extra spindles, followed by chromosome mis-segregation. Thus, drug exposure induces elevated levels of aneuploidy, which can alter the copy number of genes that improve survival in a given stress or drug. Selection then acts to increase the proportion of adaptive aneuploids in the population. Because aneuploidy is a common property of many pathogenic fungi, including those posing emerging threats to plants, animals and humans, we propose that aneuploid formation and LOH often accompanying it contribute to the rapid generation of diversity that can facilitate the emergence of fungal pathogens to new environmental niches and/or new hosts, as well as promote antifungal drug resistance that makes emerging fungal infections ever more difficult to contain.This article is part of the themed issue 'Tackling emerging fungal threats to animal health, food security and ecosystem resilience'.
Keywords: adaptation; aneuploidy; drug resistance; loss of heterozygosity; trimeras.
© 2016 The Authors.
Figures
References
-
- Institute of Medicine (US) Forum on Microbial Threats. 2011. Fungal diseases: an emerging threat to human, animal, and plant health: workshop summary. ( 10.17226/13147) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
