Anillin Phosphorylation Controls Timely Membrane Association and Successful Cytokinesis
- PMID: 28081137
- PMCID: PMC5230765
- DOI: 10.1371/journal.pgen.1006511
Anillin Phosphorylation Controls Timely Membrane Association and Successful Cytokinesis
Abstract
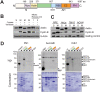
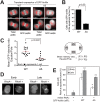
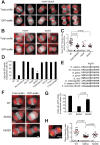
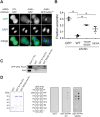
During cytokinesis, a contractile ring generates the constricting force to divide a cell into two daughters. This ring is composed of filamentous actin and the motor protein myosin, along with additional structural and regulatory proteins, including anillin. Anillin is a required scaffold protein that links the actomyosin ring to membrane and its organizer, RhoA. However, the molecular basis for timely action of anillin at cytokinesis remains obscure. Here, we find that phosphorylation regulates efficient recruitment of human anillin to the equatorial membrane. Anillin is highly phosphorylated in mitosis, and is a substrate for mitotic kinases. We surveyed function of 46 residues on anillin previously found to be phosphorylated in human cells to identify those required for cytokinesis. Among these sites, we identified S635 as a key site mediating cytokinesis. Preventing S635 phosphorylation adjacent to the AH domain disrupts anillin concentration at the equatorial cortex at anaphase, whereas a phosphomimetic mutant, S635D, partially restores this localization. Time-lapse videomicroscopy reveals impaired recruitment of S635A anillin to equatorial membrane and a transient unstable furrow followed by ultimate failure in cytokinesis. A phosphospecific antibody confirms phosphorylation at S635 in late cytokinesis, although it does not detect phosphorylation in early cytokinesis, possibly due to adjacent Y634 phosphorylation. Together, these findings reveal that anillin recruitment to the equatorial cortex at anaphase onset is enhanced by phosphorylation and promotes successful cytokinesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Developmental cell. 2003;4(1):29–39. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

