Definition of Human Epitopes Recognized in Tetanus Toxoid and Development of an Assay Strategy to Detect Ex Vivo Tetanus CD4+ T Cell Responses
- PMID: 28081174
- PMCID: PMC5230748
- DOI: 10.1371/journal.pone.0169086
Definition of Human Epitopes Recognized in Tetanus Toxoid and Development of an Assay Strategy to Detect Ex Vivo Tetanus CD4+ T Cell Responses
Erratum in
-
Correction: Definition of Human Epitopes Recognized in Tetanus Toxoid and Development of an Assay Strategy to Detect Ex Vivo Tetanus CD4+ T Cell Responses.PLoS One. 2018 Feb 20;13(2):e0193382. doi: 10.1371/journal.pone.0193382. eCollection 2018. PLoS One. 2018. PMID: 29462188 Free PMC article.
Abstract
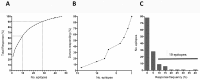
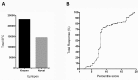
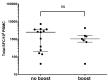
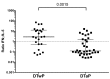
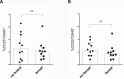
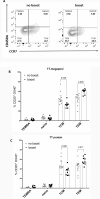
Despite widespread uses of tetanus toxoid (TT) as a vaccine, model antigen and protein carrier, TT epitopes have been poorly characterized. Herein we defined the human CD4+ T cell epitope repertoire by reevaluation of previously described epitopes and evaluation of those derived from prediction of HLA Class II binding. Forty-seven epitopes were identified following in vitro TT stimulation, with 28 epitopes accounting for 90% of the total response. Despite this diverse range of epitopes, individual responses were associated with only a few immunodominant epitopes, with each donor responding on average to 3 epitopes. For the top 14 epitopes, HLA restriction could be inferred based on HLA typing of the responding donors. HLA binding predictions re-identified the vast majority of known epitopes, and identified 24 additional novel epitopes. With these epitopes, we created a TT epitope pool, which allowed us to characterize TT responses directly ex vivo using a cytokine-independent Activation Induced Marker (AIM) assay. These TT responses were highly Th1 or Th2 polarized, which was dependent upon the original priming vaccine, either the cellular DTwP or acellular DTaP formulation. This polarization remained despite the original priming having occurred decades past and a recent booster immunization with a reduced acellular vaccine formulation. While TT responses following booster vaccination were not durably increased in magnitude, they were associated with a relative expansion of CD4+ effector memory T cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Epidemiology and Prevention of Vaccine-Preventable Diseases. The Pink Book: Course Textbook. 13th Edition2015.
-
- Tetanus vaccine: WHO position paper. Weekly epidemiological record 20. 2006;(81)(May 19):197–208.
-
- Tetanus (Lockjaw) Vaccination 2016. Available from: http://www.cdc.gov/vaccines/vpd-vac/tetanus/.
-
- Cherry JD. The present and future control of pertussis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51(6):663–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

