Randomised phase II trial of irinotecan plus S-1 in patients with gemcitabine-refractory pancreatic cancer
- PMID: 28081543
- PMCID: PMC5318973
- DOI: 10.1038/bjc.2016.436
Randomised phase II trial of irinotecan plus S-1 in patients with gemcitabine-refractory pancreatic cancer
Abstract
Background: We aimed to compare the efficacy and safety of irinotecan/S-1 (IRIS) therapy with S-1 monotherapy in patients with gemcitabine-refractory pancreatic cancer.
Methods: Patients were treated with oral S-1 (80-120 mg for 14 days every 4 weeks) plus intravenous irinotecan (100 mg m-2 on days 1 and 15 every 4 weeks; IRIS group) or oral S-1 group (80-120 mg daily for 28 days every 6 weeks). The primary endpoint was progression-free survival (PFS).
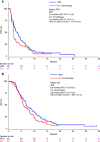
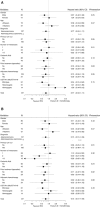
Results: Of 137 patients enrolled, 127 were eligible for efficacy. The median PFS in the IRIS group and S-1 monotherapy group were 3.5 and 1.9 months, respectively (hazard ratio (HR)=0.77; 95% confidence interval (CI), 0.53-1.11; P=0.18), while the median overall survival (OS) were 6.8 and 5.8 months, respectively (HR=0.75; 95% CI, 0.51-1.09; P=0.13). Response rate was significantly higher in the IRIS group than in the S-1 monotherapy group (18.3% vs 6.0%, P=0.03). Grade 3 or higher neutropenia and anorexia occurred more frequently in the IRIS group.
Conclusions: There was a trend for better PFS and OS in the IRIS group that could be a treatment arm in the clinical trials for gemcitabine-refractory pancreatic cancer.
Conflict of interest statement
Tatsuya Ioka has received research fund and honoraria including Speakers from Bureau from Taiho Pharmaceutical and Yakult Honsha. Tatsuya Ioka held an advisory role for Taiho Pharmace utical. Yoshito Komatsu has received research fund and honoraria from Yakult Honsha and Taiho Pharmaceutical. Nobumasa Mizuno, Haruo Iguchi, and Junji Furuse have received honoraria from Taiho Pharmaceutical and Yakult Honsha. Nobumasa Mizuno and Atsushi Ishiguro have received research fund from Taiho Pharmaceutical. Akihito Tsuji and Junji Furuse have received research fund from Taiho Pharmaceutical and Yakult Honsha. Taroh Satoh has received research fund from Yakult Honsha. Junji Furuse and Yuh Sakata held an advisory role for Taiho Pharmaceutical and Yakult Honsha. Chikuma Hamada held an advisory role for Yakult Honsha. All the remaining authors have declared no conflict of interest.
Figures

References
-
- Burris HA, Moore MI, Anderson J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvement in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol 15: 2403–2413. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreas cancer. N Engl J Med 364: 1817–1825. - PubMed
-
- Gill S, Ko YJ, Cripps M, Beaudoin A, Dhesy-Thind SK, Zulfiqar M, Zalewski P, Do T, Cano PO, Lam W, Dowden SD, Grassin H, Stewart J, Moore MJ (2016) PANCREOX: a randomized Phase III study of 5-fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 34: 3914–3920. - PubMed
-
- Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Jang IJ, Lee DH, Lee JS (2006) Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non–small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 24: 2237–2244. - PubMed
-
- Hoskins JM, Marcuello E, Altes A, Marsh S, Maxwell T, Van Booven DJ, Paré L, Culverhouse R, McLeod HL, Baiget M (2008) Irinotecan pharmacogenetics: influence of pharmacodynamic genes. Clin Cancer Res 14: 1788–1796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

