A case of levocetirizine-induced liver injury
- PMID: 28081586
- PMCID: PMC5266344
- DOI: 10.3350/cmh.2016.0023
A case of levocetirizine-induced liver injury
Abstract
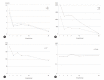
Levocetirizine is a second-generation nonsedative antihistaminic agent that has been demonstrated to be safe and effective for treating allergic disease. There was only one case report of levocetirizine-induced liver toxicity, but a liver biopsy was not performed. In this article, we present the first case of levocetirizine-induced liver injury with histologic findings. A 48-year-old man was hospitalized with jaundice and generalized pruritus that had developed after 2 months of therapy with levocetirizine for prurigo nodularis. Laboratory findings revealed acute hepatitis with cholestasis. A liver biopsy demonstrated portal inflammation and hepatitis with apoptotic hepatocytes. The patient fully recovered 3 weeks after withdrawing levocetirizine. Although levocetirizine is safe and effective, physicians should be aware of its potential hepatotoxicity.
Keywords: Cholestasis; Hepatotoxicity; Levocetirizine.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
-
- Gillard M, Christophe B, Wels B, Peck M, Massingham R, Chatelain P. H1 antagonists: receptor affinity versus selectivity. Inflamm Res. 2003;52(Suppl 1):S49–S50. - PubMed
-
- Grant JA, Riethuisen JM, Moulaert B, DeVos C. A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. Ann Allergy Asthma Immunol. 2002;88:190–197. - PubMed
-
- UCB Pharma Inc; Smyrna, Ga: 2008. Xyzal (levocetirizine dihydrochloride) [package insert]
-
- Maiti R, Rahman J, Jaida J, Allala U, Palani A. Rupatadine and levocetirizine for seasonal allergic rhinitis: a comparative study of efficacy and safety. Arch Otolaryngol Head Neck Surg. 2010;136:796–800. - PubMed
-
- Ekiz F, Yuksel I, Ekiz O, Coban S, Basar O, Yüksel O. Levocetirizine-induced hepatotoxicity in a patient with chronic urticaria. Ann Hepatol. 2011;10:237–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

