Immunogenicity of modified vaccinia virus Ankara expressing the hemagglutinin stalk domain of pandemic (H1N1) 2009 influenza virus
- PMID: 28081672
- PMCID: PMC5375617
- DOI: 10.1080/20477724.2016.1275464
Immunogenicity of modified vaccinia virus Ankara expressing the hemagglutinin stalk domain of pandemic (H1N1) 2009 influenza virus
Abstract
Background: Vaccination offers protection against influenza, although current vaccines need to be reformulated each year. The development of a broadly protective influenza vaccine would guarantee the induction of heterosubtypic immunity also against emerging influenza viruses of a novel subtype. Vaccine candidates based on the stalk region of the hemagglutinin (HA) have the potential to induce broad and persistent protection against diverse influenza A viruses.
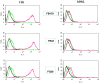
Methods: Modified vaccinia virus Ankara (MVA) expressing a headless HA (hlHA) of A/California/4/09 (CA/09) virus was used as a vaccine to immunize C57BL/6 mice. Specific antibody and cell-mediated immune responses were determined, and challenge experiments were performed by infecting vaccinated mice with CA/09 virus.
Results: Immunization of mice with CA/09-derived hlHA, vectored by MVA, was able to elicit influenza-specific broad cross-reactive antibodies and cell-mediated immune responses, but failed to induce neutralizing antibodies and did not protect mice against virus challenge.
Conclusion: Although highly immunogenic, our vaccine was unable to induce a protective immunity against influenza. A misfolded and unstable conformation of the hlHA molecule may have affected its capacity of inducing neutralizing antiviral, conformational antibodies. Design of stable hlHA-based immunogens and their delivery by recombinant MVA-based vectors has the potential of improving this promising approach for a universal influenza vaccine.
Keywords: Influenza virus; MVA vector; antibodies; vaccine.
Figures





References
-
- Bridges CB, Thompson WW, Metzer MI, et al. . Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA. 2000;284:1655–63. - PubMed
-
- Barr IG, Russell C, Besselaar TG, et al. . WHO recommendations for the viruses used in the 2013–2014 northern hemisphere influenza vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from October 2012 to January 2013. Vaccine 2014;32(37):4713–25.10.1016/j.vaccine.2014.02.014 - DOI - PubMed
-
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev. 2015;14:167–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
