ClinGen Pathogenicity Calculator: a configurable system for assessing pathogenicity of genetic variants
- PMID: 28081714
- PMCID: PMC5228115
- DOI: 10.1186/s13073-016-0391-z
ClinGen Pathogenicity Calculator: a configurable system for assessing pathogenicity of genetic variants
Abstract
Background: The success of the clinical use of sequencing based tests (from single gene to genomes) depends on the accuracy and consistency of variant interpretation. Aiming to improve the interpretation process through practice guidelines, the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) have published standards and guidelines for the interpretation of sequence variants. However, manual application of the guidelines is tedious and prone to human error. Web-based tools and software systems may not only address this problem but also document reasoning and supporting evidence, thus enabling transparency of evidence-based reasoning and resolution of discordant interpretations.
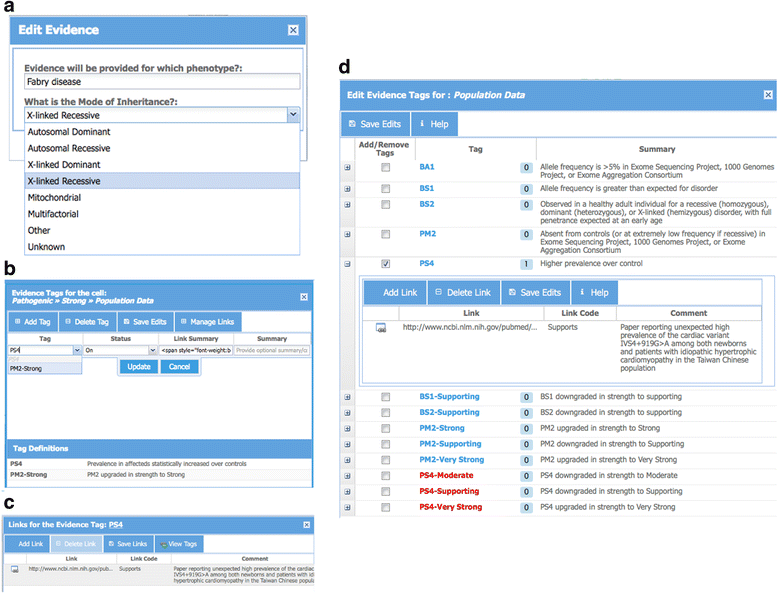
Results: In this report, we describe the design, implementation, and initial testing of the Clinical Genome Resource (ClinGen) Pathogenicity Calculator, a configurable system and web service for the assessment of pathogenicity of Mendelian germline sequence variants. The system allows users to enter the applicable ACMG/AMP-style evidence tags for a specific allele with links to supporting data for each tag and generate guideline-based pathogenicity assessment for the allele. Through automation and comprehensive documentation of evidence codes, the system facilitates more accurate application of the ACMG/AMP guidelines, improves standardization in variant classification, and facilitates collaborative resolution of discordances. The rules of reasoning are configurable with gene-specific or disease-specific guideline variations (e.g. cardiomyopathy-specific frequency thresholds and functional assays). The software is modular, equipped with robust application program interfaces (APIs), and available under a free open source license and as a cloud-hosted web service, thus facilitating both stand-alone use and integration with existing variant curation and interpretation systems. The Pathogenicity Calculator is accessible at http://calculator.clinicalgenome.org .
Conclusions: By enabling evidence-based reasoning about the pathogenicity of genetic variants and by documenting supporting evidence, the Calculator contributes toward the creation of a knowledge commons and more accurate interpretation of sequence variants in research and clinical care.
Keywords: ACMG guidelines; Big Data; ClinGen; ClinVar; Clinical Genome Resource; Clinical exome sequencing; Clinical genome sequencing; Data commons; Data sharing; Exome sequencing; Genome sequencing; Knowledge commons; Linked Data.
Figures


References
-
- Amendola LM, Jarvik GP, Leo MC, McLaughlin HM, Akkari Y, Amaral MD, et al. Performance of ACMG-AMP variant-interpretation guidelines among nine laboratories in the Clinical Sequencing Exploratory Research Consortium. Am J Hum Genet. 2016;98(6):1067–76. doi: 10.1016/j.ajhg.2016.03.024. - DOI - PMC - PubMed
-
- Harrison S, Dolinsky J, Vincent L, Chao E, Azzariti D, Das S, et al. Clinical laboratories implement the ACMG/AMP guidelines to resolve differences in variant interpretations submitted to ClinVar. In: ACMG Annual Clinical Genetics Meeting. Tampa, Florida; 2016. http://epostersonline.com/acmg2016/node/624.
-
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. doi: 10.1038/gim.2015.30. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

