Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial
- PMID: 28081952
- PMCID: PMC6108339
- DOI: 10.1016/S0140-6736(16)32410-2
Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial
Abstract
Background: Intraventricular haemorrhage is a subtype of intracerebral haemorrhage, with 50% mortality and serious disability for survivors. We aimed to test whether attempting to remove intraventricular haemorrhage with alteplase versus saline irrigation improved functional outcome.
Methods: In this randomised, double-blinded, placebo-controlled, multiregional trial (CLEAR III), participants with a routinely placed extraventricular drain, in the intensive care unit with stable, non-traumatic intracerebral haemorrhage volume less than 30 mL, intraventricular haemorrhage obstructing the 3rd or 4th ventricles, and no underlying pathology were adaptively randomly assigned (1:1), via a web-based system to receive up to 12 doses, 8 h apart of 1 mg of alteplase or 0·9% saline via the extraventricular drain. The treating physician, clinical research staff, and participants were masked to treatment assignment. CT scans were obtained every 24 h throughout dosing. The primary efficacy outcome was good functional outcome, defined as a modified Rankin Scale score (mRS) of 3 or less at 180 days per central adjudication by blinded evaluators. This study is registered with ClinicalTrials.gov, NCT00784134.
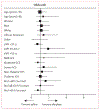
Findings: Between Sept 18, 2009, and Jan 13, 2015, 500 patients were randomised: 249 to the alteplase group and 251 to the saline group. 180-day follow-up data were available for analysis from 246 of 249 participants in the alteplase group and 245 of 251 participants in the placebo group. The primary efficacy outcome was similar in each group (good outcome in alteplase group 48% vs saline 45%; risk ratio [RR] 1·06 [95% CI 0·88-1·28; p=0·554]). A difference of 3·5% (RR 1·08 [95% CI 0·90-1·29], p=0·420) was found after adjustment for intraventricular haemorrhage size and thalamic intracerebral haemorrhage. At 180 days, the treatment group had lower case fatality (46 [18%] vs saline 73 [29%], hazard ratio 0·60 [95% CI 0·41-0·86], p=0·006), but a greater proportion with mRS 5 (42 [17%] vs 21 [9%]; RR 1·99 [95% CI 1·22-3·26], p=0·007). Ventriculitis (17 [7%] alteplase vs 31 [12%] saline; RR 0·55 [95% CI 0·31-0·97], p=0·048) and serious adverse events (114 [46%] alteplase vs 151 [60%] saline; RR 0·76 [95% CI 0·64-0·90], p=0·002) were less frequent with alteplase treatment. Symptomatic bleeding (six [2%] in the alteplase group vs five [2%] in the saline group; RR 1·21 [95% CI 0·37-3·91], p=0·771) was similar.
Interpretation: In patients with intraventricular haemorrhage and a routine extraventricular drain, irrigation with alteplase did not substantially improve functional outcomes at the mRS 3 cutoff compared with irrigation with saline. Protocol-based use of alteplase with extraventricular drain seems safe. Future investigation is needed to determine whether a greater frequency of complete intraventricular haemorrhage removal via alteplase produces gains in functional status.
Funding: National Institute of Neurological Disorders and Stroke.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Intracerebral haemorrhage: no good treatment but treatment helps.Lancet. 2017 Feb 11;389(10069):575-576. doi: 10.1016/S0140-6736(17)30002-8. Epub 2017 Jan 10. Lancet. 2017. PMID: 28081951 No abstract available.
-
JNA Journal Club.J Neurosurg Anesthesiol. 2017 Jul;29(3):354-355. doi: 10.1097/ANA.0000000000000436. J Neurosurg Anesthesiol. 2017. PMID: 28498139 No abstract available.
-
Intraventricular Thrombolytics in Intraventricular Hemorrhage: Their Role is not so Clear.Neurosurgery. 2017 May 1;80(5):N31-N33. doi: 10.1093/neuros/nyx104. Neurosurgery. 2017. PMID: 28586487 No abstract available.
References
-
- Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical Care Med 1999; 27: 617–21. - PubMed
-
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. New Engl J Med 2001; 344: 1450–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

