Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy
- PMID: 28082358
- PMCID: PMC5294852
- DOI: 10.1084/jem.20160620
Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy
Abstract
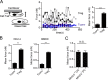
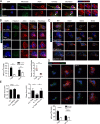
Dendritic cells are targeted by regulatory T (T reg) cells, in a manner that operates as an indirect mode of T cell suppression. In this study, using a combination of single-cell force spectroscopy and structured illumination microscopy, we analyze individual T reg cell-DC interaction events and show that T reg cells exhibit strong intrinsic adhesiveness to DCs. This increased DC adhesion reduces the ability of contacted DCs to engage other antigen-specific cells. We show that this unusually strong LFA-1-dependent adhesiveness of T reg cells is caused in part by their low calpain activities, which normally release integrin-cytoskeleton linkage, and thereby reduce adhesion. Super resolution imaging reveals that such T reg cell adhesion causes sequestration of Fascin-1, an actin-bundling protein essential for immunological synapse formation, and skews Fascin-1-dependent actin polarization in DCs toward the T reg cell adhesion zone. Although it is reversible upon T reg cell disengagement, this sequestration of essential cytoskeletal components causes a lethargic state of DCs, leading to reduced T cell priming. Our results reveal a dynamic cytoskeletal component underlying T reg cell-mediated DC suppression in a contact-dependent manner.
© 2017 Chen et al.
Figures


References
-
- Al-Alwan, M.M., Liwski R.S., Haeryfar S.M., Baldridge W.H., Hoskin D.W., Rowden G., and West K.A.. 2003. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J. Immunol. 171:4479–4483. 10.4049/jimmunol.171.9.4479 - DOI - PubMed
-
- Aldinucci, A., Rizzetto L., Pieri L., Nosi D., Romagnoli P., Biagioli T., Mazzanti B., Saccardi R., Beltrame L., Massacesi L., et al. . 2010. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. J. Immunol. 185:5102–5110. 10.4049/jimmunol.1001332 - DOI - PubMed
-
- Au-Yeung, B.B., Levin S.E., Zhang C., Hsu L.Y., Cheng D.A., Killeen N., Shokat K.M., and Weiss A.. 2010. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat. Immunol. 11:1085–1092. 10.1038/ni.1955 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

