Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains
- PMID: 28082376
- PMCID: PMC5815656
- DOI: 10.1093/hmg/ddw381
Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains
Abstract
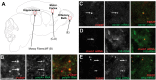
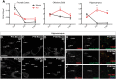
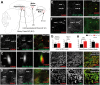
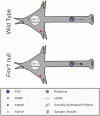
Local mRNA translation in growing axons allows for rapid and precise regulation of protein expression in response to extrinsic stimuli. However, the role of local translation in mature CNS axons is unknown. Such a mechanism requires the presence of translational machinery and associated mRNAs in circuit-integrated brain axons. Here we use a combination of genetic, quantitative imaging and super-resolution microscopy approaches to show that mature axons in the mammalian brain contain ribosomes, the translational regulator FMRP and a subset of FMRP mRNA targets. This axonal translational machinery is associated with Fragile X granules (FXGs), which are restricted to axons in a stereotyped subset of brain circuits. FXGs and associated axonal translational machinery are present in hippocampus in humans as old as 57 years. This FXG-associated axonal translational machinery is present in adult rats, even when adult neurogenesis is blocked. In contrast, in mouse this machinery is only observed in juvenile hippocampal axons. This differential developmental expression was specific to the hippocampus, as both mice and rats exhibit FXGs in mature axons in the adult olfactory system. Experiments in Fmr1 null mice show that FMRP regulates axonal protein expression but is not required for axonal transport of ribosomes or its target mRNAs. Axonal translational machinery is thus a feature of adult CNS neurons. Regulation of this machinery by FMRP could support complex behaviours in humans throughout life.
Published by Oxford University Press 2016. This work is written by US Government employees and is in the public domain in the US.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

