Effects of Ferric Citrate in Patients with Nondialysis-Dependent CKD and Iron Deficiency Anemia
- PMID: 28082519
- PMCID: PMC5461803
- DOI: 10.1681/ASN.2016101053
Effects of Ferric Citrate in Patients with Nondialysis-Dependent CKD and Iron Deficiency Anemia
Abstract
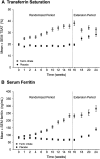
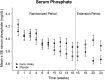
Iron deficiency anemia is common and consequential in nondialysis-dependent CKD (NDD-CKD). Efficacy and tolerability of conventional oral iron supplements are mixed; intravenous iron administration associates with finite but important risks. We conducted a randomized double-blind clinical trial in adults with NDD-CKD and iron deficiency anemia to compare the safety and efficacy of oral ferric citrate (n=117) and placebo (n=115). The primary end point was the proportion of patients who achieved a ≥1.0 g/dl increase in hemoglobin at any time during a 16-week randomized period. Patients who completed the 16-week period could also participate in an 8-week open-label extension period. Significantly more patients randomized to ferric citrate achieved the primary end point (61 [52.1%] versus 22 [19.1%] with placebo; P<0.001). All secondary end points reached statistical significance in the ferric citrate group, including the mean relative change in hemoglobin (0.84 g/dl; 95% confidence interval, 0.58 to 1.10 g/dl; P<0.001) and the proportion of patients who achieved a sustained increase in hemoglobin (≥0.75 g/dl over any 4-week period during the randomized trial; 57 [48.7%] versus 17 [14.8%] with placebo; P<0.001). Rates of serious adverse events were similar in the ferric citrate (12.0%) and placebo groups (11.2%). Gastrointestinal disorders were the most common adverse events, with diarrhea reported in 24 (20.5%) and 19 (16.4%) and constipation in 22 (18.8%) and 15 (12.9%) patients treated with ferric citrate and placebo, respectively. Overall, in patients with NDD-CKD, we found oral ferric citrate to be a safe and efficacious treatment for iron deficiency anemia.
Keywords: anemia; blood transfusion; clinical trial; hemoglobin; iron deficiency; renal dialysis.
Copyright © 2017 by the American Society of Nephrology.
Figures
References
-
- Stancu S, Stanciu A, Zugravu A, Bârsan L, Dumitru D, Lipan M, Mircescu G: Bone marrow iron, iron indices, and the response to intravenous iron in patients with non-dialysis-dependent CKD. Am J Kidney Dis 55: 639–647, 2010 - PubMed
-
- Rao M, Pereira BJ: Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int 68: 1432–1438, 2005 - PubMed
-
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 - PubMed
-
- Kidney Disease Improving Global Outcomes (KDIGO) Anemia Work Group : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

