Principles for designing proteins with cavities formed by curved β sheets
- PMID: 28082595
- PMCID: PMC5588894
- DOI: 10.1126/science.aah7389
Principles for designing proteins with cavities formed by curved β sheets
Abstract
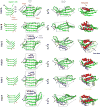
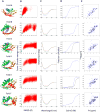
Active sites and ligand-binding cavities in native proteins are often formed by curved β sheets, and the ability to control β-sheet curvature would allow design of binding proteins with cavities customized to specific ligands. Toward this end, we investigated the mechanisms controlling β-sheet curvature by studying the geometry of β sheets in naturally occurring protein structures and folding simulations. The principles emerging from this analysis were used to design, de novo, a series of proteins with curved β sheets topped with α helices. Nuclear magnetic resonance and crystal structures of the designs closely match the computational models, showing that β-sheet curvature can be controlled with atomic-level accuracy. Our approach enables the design of proteins with cavities and provides a route to custom design ligand-binding and catalytic sites.
Copyright © 2017, American Association for the Advancement of Science.
Figures


References
-
- Röthlisberger D, Khersonsky O, Wollacott AM, Jiang L, Dechancie J, Betker J, et al. Nature. 2008;453:190–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

