Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice
- PMID: 28082598
- PMCID: PMC6075616
- DOI: 10.1093/toxsci/kfw248
Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice
Abstract
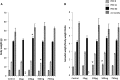
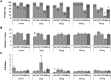
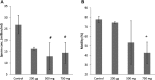
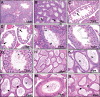
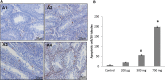
Di-(2-ethylhexyl) phthalate (DEHP) is the most commonly used phthalate, and it is an endocrine-disrupting chemical. This study tested a hypothesis that prenatal exposure to DEHP lays the foundation for premature gonadal dysfunction and subsequent reproductive senescence in male mice. Pregnant female CD-1 mice were orally dosed with vehicle control (tocopherol-stripped corn oil) or with 20 μg/kg/day, 200 μg/kg/day, 500 mg/kg/day, or 750 mg/kg/day of DEHP from gestational day 11 to birth. Overall, the prenatal DEHP exposure did not cause any overt physical health problems in male offspring, as no significant differences in their body nor gonadal weight were seen up to the age of 23 months. However, an age- and dose-dependent gonadal dysfunction was observed. As early as 7 months of age, the 750 mg/kg/day group of mice exhibited significantly reduced fertility. At 19 months of age, 86% of the 750 mg/kg/day mice became infertile, whereas only 25% of the control mice were infertile. At this age, all of the DEHP-exposed mice had lower serum testosterone levels, higher serum estradiol levels, and higher LH levels compared with control mice. Histological evaluations showed that mice prenatally exposed to DEHP displayed a wide array of gonadal and epididymal abnormalities such as increased germ cell apoptosis, degenerative seminiferous tubules, oligozoospermia, asthenozoospermia, and teratozoospermia in comparison to age-matching control mice. In summary, this study shows that prenatal exposure to DEHP induces premature reproductive senescence in male mice.
Keywords: DEHP; endocrine disruptor; fertility; semen.; testes.
© The Author 2017. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Agency for Toxic Substances and Disease Registry ( 2002). Toxicological profile: di(2-ethylhexyl)phthalate (DEHP). ATSDR, Atlanta, GA. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp9.html. - PubMed
-
- Andrade A. J., Grande S. W., Talsness C. E., Grote K., Golombiewski A., Sterner-Kock A., Chahoud I. (2006). A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology 225, 64–74. - PubMed
-
- Atanassova N., McKinnell C., Fisher J., Sharpe R. M. (2005). Neonatal treatment of rats with diethylstilboestrol (DES) induces stromal-epithelial abnormalities of the vas deferens and cauda epididymis in adulthood following delayed basal cell development. Reproduction 129, 589–601. - PubMed
-
- Barlow N. J., Foster P. M. D. (2003). Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol. 31, 397–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

