Epigenetic regulation and chromatin remodeling in learning and memory
- PMID: 28082740
- PMCID: PMC5291841
- DOI: 10.1038/emm.2016.140
Epigenetic regulation and chromatin remodeling in learning and memory
Abstract
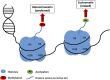
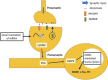
Understanding the underlying mechanisms of memory formation and maintenance has been a major goal in the field of neuroscience. Memory formation and maintenance are tightly controlled complex processes. Among the various processes occurring at different levels, gene expression regulation is especially crucial for proper memory processing, as some genes need to be activated while some genes must be suppressed. Epigenetic regulation of the genome involves processes such as DNA methylation and histone post-translational modifications. These processes edit genomic properties or the interactions between the genome and histone cores. They then induce structural changes in the chromatin and lead to transcriptional changes of different genes. Recent studies have focused on the concept of chromatin remodeling, which consists of 3D structural changes in chromatin in relation to gene regulation, and is an important process in learning and memory. In this review, we will introduce three major epigenetic processes involved in memory regulation: DNA methylation, histone methylation and histone acetylation. We will also discuss general mechanisms of long-term memory storage and relate the epigenetic control of learning and memory to chromatin remodeling. Finally, we will discuss how epigenetic mechanisms can contribute to the pathologies of neurological disorders and cause memory-related symptoms.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

