Biologics for Targeting Inflammatory Cytokines, Clinical Uses, and Limitations
- PMID: 28083070
- PMCID: PMC5204077
- DOI: 10.1155/2016/9259646
Biologics for Targeting Inflammatory Cytokines, Clinical Uses, and Limitations
Abstract
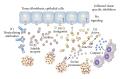
Proinflammatory cytokines are potent mediators of numerous biological processes and are tightly regulated in the body. Chronic uncontrolled levels of such cytokines can initiate and derive many pathologies, including incidences of autoimmunity and cancer. Therefore, therapies that regulate the activity of inflammatory cytokines, either by supplementation of anti-inflammatory recombinant cytokines or by neutralizing them by using blocking antibodies, have been extensively used over the past decades. Over the past few years, new innovative biological agents for blocking and regulating cytokine activities have emerged. Here, we review some of the most recent approaches of cytokine targeting, focusing on anti-TNF antibodies or recombinant TNF decoy receptor, recombinant IL-1 receptor antagonist (IL-1Ra) and anti-IL-1 antibodies, anti-IL-6 receptor antibodies, and TH17 targeting antibodies. We discuss their effects as biologic drugs, as evaluated in numerous clinical trials, and highlight their therapeutic potential as well as emphasize their inherent limitations and clinical risks. We suggest that while systemic blocking of proinflammatory cytokines using biological agents can ameliorate disease pathogenesis and progression, it may also abrogate the hosts defense against infections. Moreover, we outline the rational need to develop new therapies, which block inflammatory cytokines only at sites of inflammation, while enabling their function systemically.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures
References
-
- Atkins M. B., Lotze M. T., Dutcher J. P., et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of Clinical Oncology. 1999;17(7):2105–2116. - PubMed
-
- Doyle M. V., Lee M. T., Fong S. Comparison of the biological activities of human recombinant interleukin-2125 and native interleukin-2. Journal of Biological Response Modifiers. 1985;4(1):96–109. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

