Structural enzymology using X-ray free electron lasers
- PMID: 28083542
- PMCID: PMC5178802
- DOI: 10.1063/1.4972069
Structural enzymology using X-ray free electron lasers
Abstract
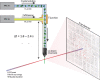
Mix-and-inject serial crystallography (MISC) is a technique designed to image enzyme catalyzed reactions in which small protein crystals are mixed with a substrate just prior to being probed by an X-ray pulse. This approach offers several advantages over flow cell studies. It provides (i) room temperature structures at near atomic resolution, (ii) time resolution ranging from microseconds to seconds, and (iii) convenient reaction initiation. It outruns radiation damage by using femtosecond X-ray pulses allowing damage and chemistry to be separated. Here, we demonstrate that MISC is feasible at an X-ray free electron laser by studying the reaction of M. tuberculosis ß-lactamase microcrystals with ceftriaxone antibiotic solution. Electron density maps of the apo-ß-lactamase and of the ceftriaxone bound form were obtained at 2.8 Å and 2.4 Å resolution, respectively. These results pave the way to study cyclic and non-cyclic reactions and represent a new field of time-resolved structural dynamics for numerous substrate-triggered biological reactions.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

