Type I Protein Secretion-Deceptively Simple yet with a Wide Range of Mechanistic Variability across the Family
- PMID: 28084193
- PMCID: PMC11575716
- DOI: 10.1128/ecosalplus.ESP-0019-2015
Type I Protein Secretion-Deceptively Simple yet with a Wide Range of Mechanistic Variability across the Family
Abstract
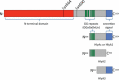
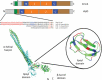
A very large type I polypeptide begins to reel out from a ribosome; minutes later, the still unidentifiable polypeptide, largely lacking secondary structure, is now in some cases a thousand or more residues longer. Synthesis of the final hundred C-terminal residues commences. This includes the identity code, the secretion signal within the last 50 amino acids, designed to dock with a waiting ATP binding cassette (ABC) transporter. What happens next is the subject of this review, with the main, but not the only focus on hemolysin HlyA, an RTX protein toxin secreted by the type I system. Transport substrates range from small peptides to giant proteins produced by many pathogens. These molecules, without detectable cellular chaperones, overcome enormous barriers, crossing two membranes before final folding on the cell surface, involving a unique autocatalytic process.Unfolded HlyA is extruded posttranslationally, C-terminal first. The transenvelope "tunnel" is formed by HlyB (ABC transporter), HlyD (membrane fusion protein) straddling the inner membrane and periplasm and TolC (outer membrane). We present a new evaluation of the C-terminal secretion code, and the structure function of HlyD and HlyB at the heart of this nanomachine. Surprisingly, key details of the secretion mechanism are remarkably variable in the many type I secretion system subtypes. These include alternative folding processes, an apparently distinctive secretion code for each type I subfamily, and alternative forms of the ABC transporter; most remarkably, the ABC protein probably transports peptides or polypeptides by quite different mechanisms. Finally, we suggest a putative structure for the Hly-translocon, HlyB, the multijointed HlyD, and the TolC exit.
Figures






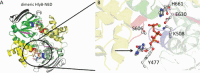
References
-
- Higgins CF, Hiles ID, Salmond GP, Gill DR, Downie JA, Evans IJ, Holland IB, Gray L, Buckel SD, Bell AW, et al. 1986. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323:448–450. - PubMed
-
- Welch RA. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol 5:521–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

