Oxygen activation by mononuclear nonheme iron dioxygenases involved in the degradation of aromatics
- PMID: 28084551
- PMCID: PMC5360381
- DOI: 10.1007/s00775-017-1436-5
Oxygen activation by mononuclear nonheme iron dioxygenases involved in the degradation of aromatics
Abstract
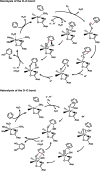
Molecular oxygen is utilized in numerous metabolic pathways fundamental for life. Mononuclear nonheme iron-dependent oxygenase enzymes are well known for their involvement in some of these pathways, activating O2 so that oxygen atoms can be incorporated into their primary substrates. These reactions often initiate pathways that allow organisms to use stable organic molecules as sources of carbon and energy for growth. From the myriad of reactions in which these enzymes are involved, this perspective recounts the general mechanisms of aromatic dihydroxylation and oxidative ring cleavage, both of which are ubiquitous chemical reactions found in life-sustaining processes. The organic substrate provides all four electrons required for oxygen activation and insertion in the reactions mediated by extradiol and intradiol ring-cleaving catechol dioxygenases. In contrast, two of the electrons are provided by NADH in the cis-dihydroxylation mechanism of Rieske dioxygenases. The catalytic nonheme Fe center, with the aid of active site residues, facilitates these electron transfers to O2 as key elements of the activation processes. This review discusses some general questions for the catalytic strategies of oxygen activation and insertion into aromatic compounds employed by mononuclear nonheme iron-dependent dioxygenases. These include: (1) how oxygen is activated, (2) whether there are common intermediates before oxygen transfer to the aromatic substrate, and (3) are these key intermediates unique to mononuclear nonheme iron dioxygenases?
Keywords: Catalytic strategies; Crystal structure; High-valent iron species; Metabolism; Nonheme iron enzymes; Oxidative degradation; Reactive oxygen species; Ring-cleaving dioxygenase; Spectroscopy.
Figures




References
-
- Hayaishi O, Katagiri M, Rothberg S. Mechanism of the pyrocatechase reaction. J Am Chem Soc. 1955;77:5450–5451.
-
- Mason HS, Fowlks WL, Peterson E. Oxygen transfer and electron transport by the phenolase complex. J Am Chem Soc. 1955;77:2914–2915.
-
- Hayaishi O. From oxygenase to sleep. J Biol Chem. 2008;283:19165–19175. - PubMed
-
- Hayaishi O, Rothberg S, Mehler AH, Saito Y. Studies on oxygenases; enzymatic formation of kynurenine from tryptophan. J Biol Chem. 1957;229:889–896. - PubMed
-
- Geng J, Liu A. Heme-dependent dioxygenases in tryptophan oxidation. Arch Biochem Biophys. 2014;544:18–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

