Rubisco small subunits from the unicellular green alga Chlamydomonas complement Rubisco-deficient mutants of Arabidopsis
- PMID: 28084636
- PMCID: PMC5363358
- DOI: 10.1111/nph.14414
Rubisco small subunits from the unicellular green alga Chlamydomonas complement Rubisco-deficient mutants of Arabidopsis
Abstract
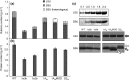
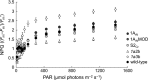
Introducing components of algal carbon concentrating mechanisms (CCMs) into higher plant chloroplasts could increase photosynthetic productivity. A key component is the Rubisco-containing pyrenoid that is needed to minimise CO2 retro-diffusion for CCM operating efficiency. Rubisco in Arabidopsis was re-engineered to incorporate sequence elements that are thought to be essential for recruitment of Rubisco to the pyrenoid, namely the algal Rubisco small subunit (SSU, encoded by rbcS) or only the surface-exposed algal SSU α-helices. Leaves of Arabidopsis rbcs mutants expressing 'pyrenoid-competent' chimeric Arabidopsis SSUs containing the SSU α-helices from Chlamydomonas reinhardtii can form hybrid Rubisco complexes with catalytic properties similar to those of native Rubisco, suggesting that the α-helices are catalytically neutral. The growth and photosynthetic performance of complemented Arabidopsis rbcs mutants producing near wild-type levels of the hybrid Rubisco were similar to those of wild-type controls. Arabidopsis rbcs mutants expressing a Chlamydomonas SSU differed from wild-type plants with respect to Rubisco catalysis, photosynthesis and growth. This confirms a role for the SSU in influencing Rubisco catalytic properties.
Keywords: Arabidopsis thaliana; Chlamydomonas reinhardtii; Rubisco; carbon concentrating mechanism (CCM); chloroplast; photosynthesis; pyrenoid; tobacco.
© 2017 The Authors. New Phytologist © 2017 New Phytologist Trust.
Figures

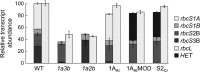


Comment in
-
A step forward to building an algal pyrenoid in higher plants.New Phytol. 2017 Apr;214(2):496-499. doi: 10.1111/nph.14514. New Phytol. 2017. PMID: 28318030 No abstract available.
References
-
- Andriotis VM, Pike MJ, Bunnewell S, Hills MJ, Smith AM. 2010. The plastidial glucose‐6‐phosphate/phosphate antiporter GPT1 is essential for morphogenesis in Arabidopsis embryos. Plant Journal 64: 128–139. - PubMed
-
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast‐based CO2‐concentrating mechanisms in algae. Canadian Journal of Botany 76: 1052–1071.
-
- Bellasio C, Beerling DJ, Griffiths H. 2016. An Excel tool for deriving key photosynthetic parameters from combined gas exchange and chlorophyll fluorescence: theory and practice. Plant, Cell & Environment 39: 1180–1197. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

