Age-Dependent Changes in AMPK Metabolic Pathways in the Lung in a Mouse Model of Hemorrhagic Shock
- PMID: 28085510
- PMCID: PMC5449487
- DOI: 10.1165/rcmb.2016-0118OC
Age-Dependent Changes in AMPK Metabolic Pathways in the Lung in a Mouse Model of Hemorrhagic Shock
Abstract
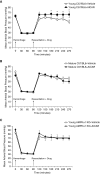
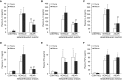
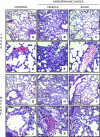
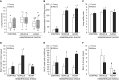
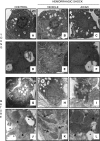
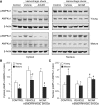
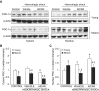
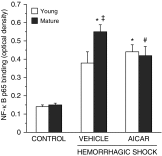
The development of multiple organ failure in patients with hemorrhagic shock is significantly influenced by patient age. Adenosine monophosphate-activated protein kinase (AMPK) is a crucial regulator of energy homeostasis, which coordinates metabolic repair during cellular stress. We investigated whether AMPK-regulated signaling pathways are age-dependent in hemorrhage-induced lung injury and whether AMPK activation by 5-amino-4-imidazole carboxamide riboside (AICAR) affords lung protective effects. Male C57/BL6 young mice (3-5 mo), mature adult mice (9-12 mo), and young AMPKα1 knockout mice (3-5 mo) were subjected to hemorrhagic shock by blood withdrawing, followed by resuscitation with shed blood and lactated Ringer's solution. Plasma proinflammatory cytokines were similarly elevated in C57/BL6 young and mature adult mice after hemorrhagic shock. However, mature adult mice exhibited more severe lung edema and neutrophil infiltration, and higher mitochondrial damage in alveolar epithelial type II cells, than did young mice. No change in autophagy was observed. At molecular analysis, the phosphorylation of the catalytic subunit AMPKα1 was associated with nuclear translocation of peroxisome proliferator-activated receptor γ co-activator-α in young, but not mature, adult mice. Treatment with AICAR ameliorated the disruption of lung architecture in mice of both ages; however, effects in mature adult mice were different than young mice and also involved inhibition of nuclear factor-κB. In young AMPKα1 knockout mice, AICAR failed to improve hypotension and lung neutrophil infiltration. Our data demonstrate that during hemorrhagic shock, AMPK-dependent metabolic repair mechanisms are important for mitigating lung injury. However, these mechanisms are less competent with age.
Keywords: AICAR; AMPK; autophagy; hemorrhagic shock; mitochondria.
Figures



References
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. - PubMed
-
- Lehmann R, Beekley A, Casey L, Salim A, Martin M. The impact of advanced age on trauma triage decisions and outcomes: a statewide analysis. Am J Surg. 2009;197:571–574, discussion 574–575. - PubMed
-
- Dewar DC, Tarrant SM, King KL, Balogh ZJ. Changes in the epidemiology and prediction of multiple-organ failure after injury. J Trauma Acute Care Surg. 2013;74:774–779. - PubMed
-
- Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, et al. Inflammation and Host Response to Injury Investigators. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19:77. - PMC - PubMed
-
- Cairns CB, Moore FA, Haenel JB, Gallea BL, Ortner JP, Rose SJ, Moore EE. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma. 1997;42:532–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

