Photoelectric Dye Used for Okayama University-Type Retinal Prosthesis Reduces the Apoptosis of Photoreceptor Cells
- PMID: 28085534
- PMCID: PMC5385417
- DOI: 10.1089/jop.2016.0093
Photoelectric Dye Used for Okayama University-Type Retinal Prosthesis Reduces the Apoptosis of Photoreceptor Cells
Abstract
Purpose: Our previous study demonstrated that photoelectric dye-coupled polyethylene film (Okayama University-type retinal prosthesis), which was implanted in subretinal space of the eyes of Royal College of Surgeons (RCS) rats, prevented retinal neurons from apoptotic death. In this study, we aimed to examine whether photoelectric dye itself would protect retinal neurons from apoptosis in RCS rats.
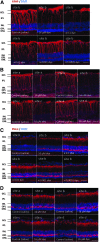
Methods: RCS rats received intravitreous injection of different concentrations of the dye in the left eye and housed under a 12-h light-dark cycle. Saline injection in the right eye served as control. In addition, RCS rats with dye injection were kept in 24-h daily dark condition. Sections were processed for terminal deoxynucleotidyl transferase-mediated fluorescein-conjugated-dUTP nick-end-labeling (TUNEL) assay and immunohistochemical staining of glial fibrillary acidic protein (GFAP) and protein kinase Cα (PKCα).
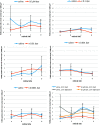
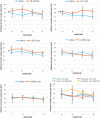
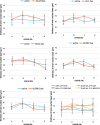
Results: The number of TUNEL-positive cells significantly decreased in the retina of dye-injected eyes compared with those in saline-injected eyes (P = 0.0001, 2-factor analysis of variance [ANOVA]), under 12-h light-dark cycle. Significant decrease of TUNEL-positive cells was noted in the retina of rats with dye injection compared with those with saline injection, kept under 24-h dark condition (P = 0.0001, 2-factor ANOVA). Immunoreactive area for GFAP decreased significantly in the retina of dye-injected eyes compared with that in controls (P = 0.0001, 2-factor ANOVA), whereas immunoreactive area for PKCα increased significantly in the retina of dye-injected eyes compared with that in controls (P = 0.01, 2-factor ANOVA).
Conclusions: Photoelectric dye inhibits apoptotic death of photoreceptor cells in RCS rats and downregulates GFAP expression in retinal Müller cells. Photoelectric dye may be a candidate agent for neuroprotection in retinitis pigmentosa and other retinal diseases.
Keywords: GFAP; PKCα; apoptosis; drug; photoreceptors; retina.
Conflict of interest statement
No competing financial interests exist.
Figures





References
-
- Loewenstein J.I., Montezuma S.R., and Rizzo J.F. III. Outer retinal degeneration: an electronic retinal prosthesis as a treatment strategy. Arch. Ophthalmol. 122:587–596, 2004 - PubMed
-
- Tamaki M., and Matsuo T. Optical coherence tomographic parameters as objective signs for visual acuity in patients with retinitis pigmentosa, future candidates for retinal prostheses. J. Artif. Organs. 14:140–150, 2011 - PubMed
-
- Zrenner E. Will retinal implants restore vision? Science. 295:1022–1025, 2002 - PubMed
-
- Chow A.Y., Chow V.Y., Packo K.H., et al. The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch. Ophthalmol. 122:460–469, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
