MicroRNA-432 targeting E2F3 and P55PIK inhibits myogenesis through PI3K/AKT/mTOR signaling pathway
- PMID: 28085550
- PMCID: PMC5367255
- DOI: 10.1080/15476286.2017.1279786
MicroRNA-432 targeting E2F3 and P55PIK inhibits myogenesis through PI3K/AKT/mTOR signaling pathway
Abstract
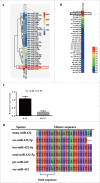
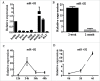
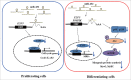
Skeletal muscle is the dominant executant in locomotion and regulator in energy metabolism. Embryonic myogenesis and postnatal muscle growth are controlled by a cascade of transcription factors and epigenetic regulatory mechanisms. MicroRNAs (miRNAs), a family of non-coding RNA of 22 nucleotides in length, post-transcriptionally regulates expression of mRNA by pairing the seed sequence to 3' UTR of target mRNA. Increasing evidence has demonstrated that miRNAs are important regulators in diverse myogenic processes. The profiling of miRNA expression revealed that miR-432 is more enriched in the longissimus dorsi of 35-day-old piglets than that of adult pigs. Our gain of function study showed that miR-432 can negatively regulate both myoblast proliferation and differentiation. Mechanically, we found that miR-432 is able to down-regulate E2F transcription factor 3 (E2F3) to inactivate the expression of cell cycle and myogenic genes. We also identified that phosphatidylinositol 3-kinase regulatory subunit (P55PIK) is another target gene of miR-432 in muscle cells. downregulation of P55PIK by miR-432 leads to inhibition of P55PIK-mediated PI3K/AKT/mTOR signaling pathway during differentiation. The blocking effect of miR-432 on this pathway can be rescued by insulin treatment. Taken together, our findings identified microRNA-432 as a potent inhibitor of myogenesis which functions by targeting E2F3 and P55PIK in muscle cells.
Keywords: E2F3; P55PIK; PI3K/Akt/mTOR pathway; microRNA-432; myogenesis.
Figures





References
-
- Massie B, Conway M, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G, Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: relation to clinical severity and blood flow. Circulation 1987; 76:1009-19; PMID:3664989; https://doi.org/ 10.1161/01.CIR.76.5.1009 - DOI - PubMed
-
- Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 1990; 86:1423-27; PMID:2243122; http://dx.doi.org/ 10.1172/JCI114857 - DOI - PMC - PubMed
-
- Saltin B, Gollnick PD. Skeletal Muscle Adaptability: Significance for Metabolism and Performance. Comprehensive Physiology 2011; 555-631.
-
- Buckingham M. Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 2001; 11:440-48; PMID:11448631 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
