European Aedes albopictus and Culex pipiens Are Competent Vectors for Japanese Encephalitis Virus
- PMID: 28085881
- PMCID: PMC5268654
- DOI: 10.1371/journal.pntd.0005294
European Aedes albopictus and Culex pipiens Are Competent Vectors for Japanese Encephalitis Virus
Abstract
Background: Japanese encephalitis virus (JEV) is the causative agent of Japanese encephalitis, the leading cause of viral encephalitis in Asia. JEV transmission cycle involves mosquitoes and vertebrate hosts. The detection of JEV RNA in a pool of Culex pipiens caught in 2010 in Italy raised the concern of a putative emergence of the virus in Europe. We aimed to study the vector competence of European mosquito populations, such as Cx. pipiens and Aedes albopictus for JEV genotypes 3 and 5.
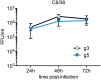
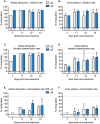
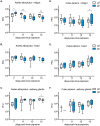
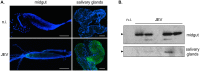
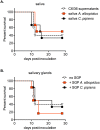
Findings: After oral feeding on an infectious blood meal, mosquitoes were dissected at various times post-virus exposure. We found that the peak for JEV infection and transmission was between 11 and 13 days post-virus exposure. We observed a faster dissemination of both JEV genotypes in Ae. albopictus mosquitoes, when compared with Cx. pipiens mosquitoes. We also dissected salivary glands and collected saliva from infected mosquitoes and showed that Ae. albopictus mosquitoes transmitted JEV earlier than Cx. pipiens. The virus collected from Ae. albopictus and Cx. pipiens saliva was competent at causing pathogenesis in a mouse model for JEV infection. Using this model, we found that mosquito saliva or salivary glands did not enhance the severity of the disease.
Conclusions: In this study, we demonstrated that European populations of Ae. albopictus and Cx. pipiens were efficient vectors for JEV transmission. Susceptible vertebrate species that develop high viremia are an obligatory part of the JEV transmission cycle. This study highlights the need to investigate the susceptibility of potential JEV reservoir hosts in Europe, notably amongst swine populations and local water birds.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Fischer M, Hills S, Staples E, Johnson B, Yaich M, Solomon T, et al. Japanese encephalitis prevention and control: advances, challenges, and new initiatives,. In: W M H S, H JM, editors. Emerging infections. 8. Washington, DC: American Society for Microbiology; 2008. p. 93–124.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

