Structures of the Human HCN1 Hyperpolarization-Activated Channel
- PMID: 28086084
- PMCID: PMC5496774
- DOI: 10.1016/j.cell.2016.12.023
Structures of the Human HCN1 Hyperpolarization-Activated Channel
Abstract
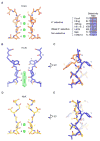
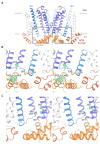
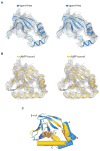
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels underlie the control of rhythmic activity in cardiac and neuronal pacemaker cells. In HCN, the polarity of voltage dependence is uniquely reversed. Intracellular cyclic adenosine monophosphate (cAMP) levels tune the voltage response, enabling sympathetic nerve stimulation to increase the heart rate. We present cryo-electron microscopy structures of the human HCN channel in the absence and presence of cAMP at 3.5 Å resolution. HCN channels contain a K+ channel selectivity filter-forming sequence from which the amino acids create a unique structure that explains Na+ and K+ permeability. The voltage sensor adopts a depolarized conformation, and the pore is closed. An S4 helix of unprecedented length extends into the cytoplasm, contacts the C-linker, and twists the inner helical gate shut. cAMP binding rotates cytoplasmic domains to favor opening of the inner helical gate. These structures advance understanding of ion selectivity, reversed polarity gating, and cAMP regulation in HCN channels.
Keywords: atomic structure; cardiac and neuronal pacemaker; cryo-electron microscopy; hyperpolarization-activated ion channel; ion selectivity; rhythmic firing; voltage-dependent gating.
Published by Elsevier Inc.
Figures

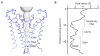

References
-
- Afonine PV, Headd JJ, Terwilliger TC, Adams PD. New tool: phenix.real_space_refine. Comput Crystallogr Newsl. 2013;4:43–44.
-
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. - PubMed
-
- Benarroch EE. HCN channels: function and clinical implications. Neurology. 2013;80:304–310. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

