Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence
- PMID: 28086196
- PMCID: PMC5228102
- DOI: 10.1016/j.redox.2016.12.001
Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence
Abstract
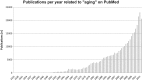
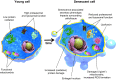
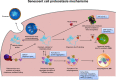
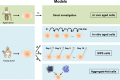
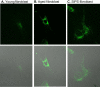
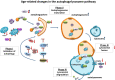
Aging is a complex phenomenon and its impact is becoming more relevant due to the rising life expectancy and because aging itself is the basis for the development of age-related diseases such as cancer, neurodegenerative diseases and type 2 diabetes. Recent years of scientific research have brought up different theories that attempt to explain the aging process. So far, there is no single theory that fully explains all facets of aging. The damage accumulation theory is one of the most accepted theories due to the large body of evidence found over the years. Damage accumulation is thought to be driven, among others, by oxidative stress. This condition results in an excess attack of oxidants on biomolecules, which lead to damage accumulation over time and contribute to the functional involution of cells, tissues and organisms. If oxidative stress persists, cellular senescence is a likely outcome and an important hallmark of aging. Therefore, it becomes crucial to understand how senescent cells function and how they contribute to the aging process. This review will cover cellular senescence features related to the protein pool such as morphological and molecular hallmarks, how oxidative stress promotes protein modifications, how senescent cells cope with them by proteostasis mechanisms, including antioxidant enzymes and proteolytic systems. We will also highlight the nutritional status of senescent cells and aged organisms (including human clinical studies) by exploring trace elements and micronutrients and on their importance to develop strategies that might increase both, life and health span and postpone aging onset.
Keywords: Aging; Antioxidants; Micronutrients; Protein oxidation; Proteostasis; Senescence; Trace elements.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

