Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers
- PMID: 28086768
- PMCID: PMC5237220
- DOI: 10.1186/s12906-016-1540-z
Probiotic mixture improves fatty liver disease by virtue of its action on lipid profiles, leptin, and inflammatory biomarkers
Abstract
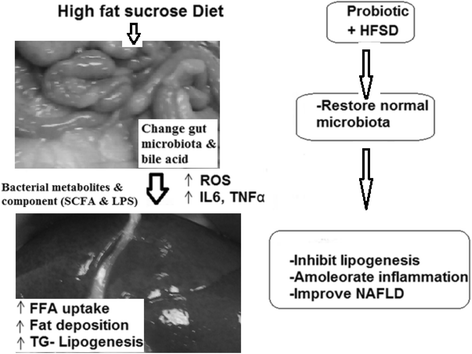
Background: A high fat diet has an essential role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). This condition is characterized by hepatic fat accumulation (steatosis) and is associated with obesity, diabetes, and fibrosis or cirrhosis of the liver. Probiotics may be useful in the treatment of steatosis. This study examined the effects of an ingested probiotic formulation on the lipid profiles, liver functions, leptin levels, and inflammatory marker levels of rats with NAFLD that had been induced via high fat and sucrose diet (HFSD).
Methods: Young male albino rats were randomly divided into three groups: a control group that was fed a standard diet; a second group that was fed a HFSD; and a third group that was given both a HFSD and ingestible probiotic mixtures. The groups were fed these diets for 16 weeks, and were then examined.
Results: HFSD-only rats showed hypertriglyceridemia, hypercholesterolemia, and elevated low density lipoprotein (LDL) levels, and their serum alanine transaminase (ALT) and bilirubin levels were significantly higher than those of the control group. Compared to rats on the standard diet, HFSD-only rats showed higher levels of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), increased serum leptin levels, and increased resistin hormone levels in the adipose tissues. In the third group, the inclusion of the probiotic mixture seemed to ameliorate the effects of the HFSD diet. The NAFD + probiotics group showed improved lipid profiles, better leptin and resistin levels, and better TNF-α and IL-6 levels than the NAFD-only group. They also showed no signs of NAFLD.
Conclusions: The probiotic mixture showed promise as a treatment for NAFLD pathogenesis, and may improve HFSD-induced steatosis through its effects on leptin, resistin, inflammatory biomarkers, and hepatic function markers. We also established that gut microbiota-mediated regulation of lipid profiles was dependent on dietary lipids and carbohydrates.
Keywords: HFSD; Inflammation; Leptin; Lipid profile; NAFLD biomarkers; Probiotics.
Figures

References
-
- Caballeria L, Auladell MA, Toran P, Miranda D, Aznar J, Pera G, et al. Prevalence and factors associated with the presence of non-alcoholic fatty liver disease in an apparently healthy adult population in primary care units. BMC Gastroenterol. 2007;7:41. doi: 10.1186/1471-230X-7-41. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

