Cheongsangbangpung-tang ameliorated the acute inflammatory response via the inhibition of NF-κB activation and MAPK phosphorylation
- PMID: 28086859
- PMCID: PMC5237186
- DOI: 10.1186/s12906-016-1501-6
Cheongsangbangpung-tang ameliorated the acute inflammatory response via the inhibition of NF-κB activation and MAPK phosphorylation
Abstract
Background: Cheongsangbangpung-tang (CBT) is a traditional herbal formula used in Eastern Asia to treat heat-related diseases and swellings in the skin. The present study was conducted to evaluate the anti-inflammatory effects of cheongsangbangpung-tang extract (CBTE) both in vitro and in vivo.
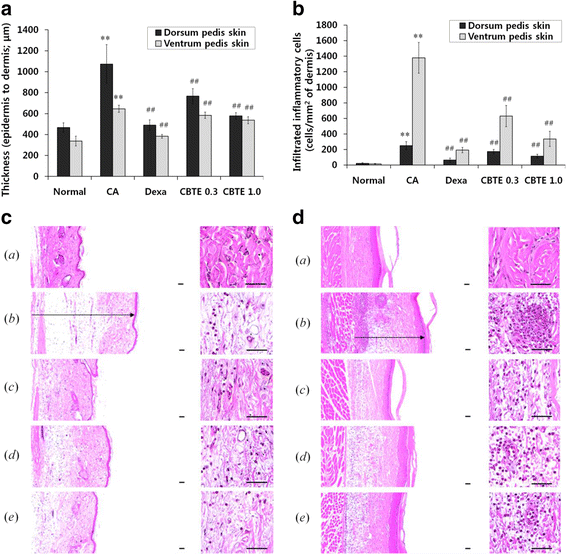
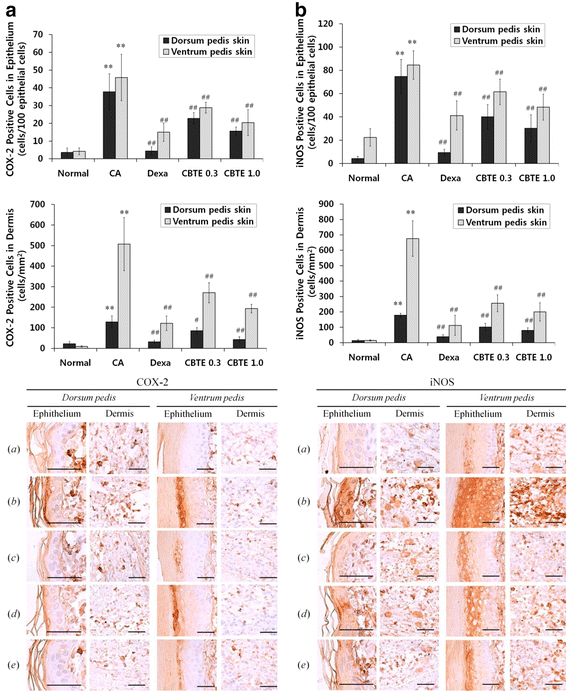
Methods: The in vitro effects of CBTE on the lipopolysaccharide (LPS)-induced production of inflammation-related proteins were examined in RAW 264.7 cells. The levels of nitric oxide (NO) were measured with the Griess reagent. Inflammatory cytokines and prostaglandin E2 (PGE2) were detected using the enzyme-linked immunosorbent assay (ELISA) method. Inflammation-related proteins were detected by Western blot. The effect of CBTE on acute inflammation in vivo was evaluated using carrageenan (CA)-induced paw oedema. To evaluate the anti-inflammatory effect, paw oedema volume, thickness of the dorsum and ventrum pedis skin, number of infiltrated inflammatory cells, and number of COX-2-, iNOS-immunoreactive cells were measured.
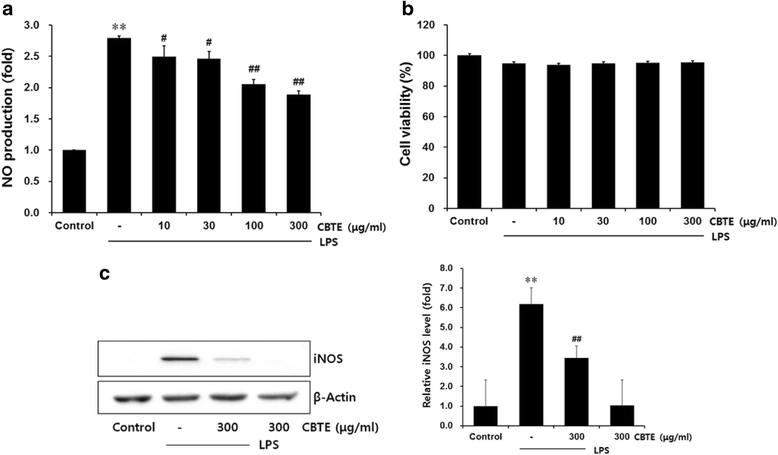
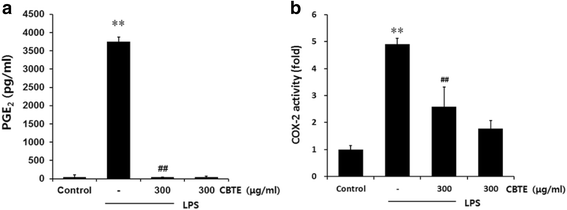
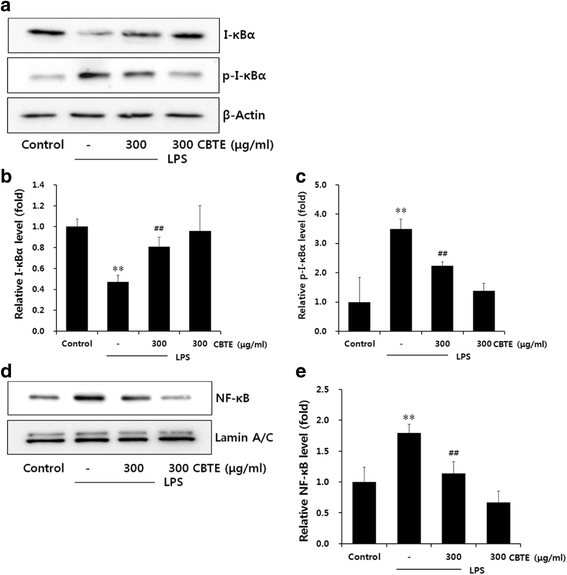
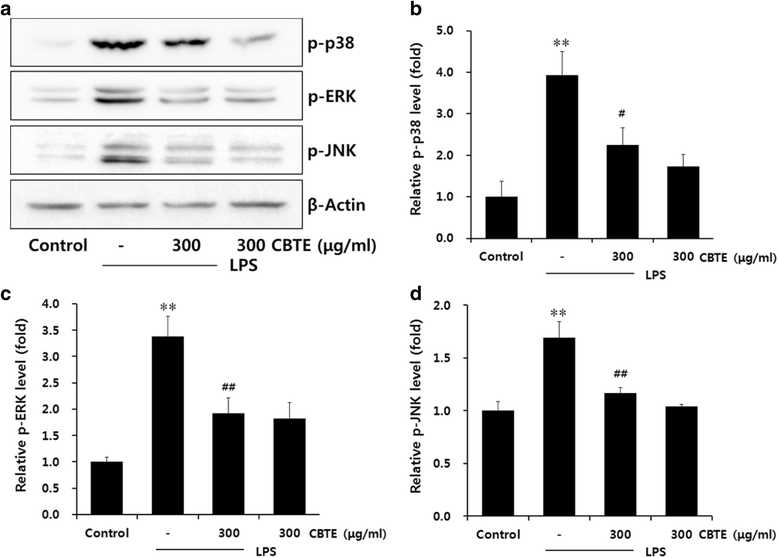
Results: In an in vitro study, CBTE inhibited the production of NO and PGE2 and also decreased the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) activity, interleukin (IL)-1β, IL-6 and tumuor necrosis factor-α. In LPS-activated macrophages, nuclear factor-kappaB (NF-κB) and mitogen-activated protein kinase (MAPK) signalling is a pivotal pathway in the inflammatory process. These plausible molecular mechanisms increased the phosphorylation of I-κBα, while the activation of NF-κB and the phosphorylation of MAPK by LPS were blocked by CBTE treatment. In our in vivo study, a CA-induced acute oedematous paw inflammation rat model was used to evaluate the anti-inflammatory effect of CBTE. CBTE significantly reduced the increases in paw swelling, skin thicknesses, infiltrated inflammatory cells and iNOS-, COX-2 positive cells induced by CA injection.
Conclusions: Based on these results, CBTE should favourably inhibit the acute inflammatory response through modulation of NF-κB activation and MAPK phosphorylation. Furthermore, the inhibition of CBTE in rat paw oedema induced by CA is considered to be clear evidence that CBTE may be a useful source to treat inflammation.
Keywords: Cheongsangbangpung-tang; Inflammation; Mitogen-activated protein kinase; Nuclear factor-kappaB; Paw oedema.
Figures



References
-
- Heo J. Donguibogam (in Korean) Seoul: Namsandang; 1983. p. 211.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

