Harnessing the early post-injury inflammatory responses for cardiac regeneration
- PMID: 28086885
- PMCID: PMC5237143
- DOI: 10.1186/s12929-017-0315-2
Harnessing the early post-injury inflammatory responses for cardiac regeneration
Abstract
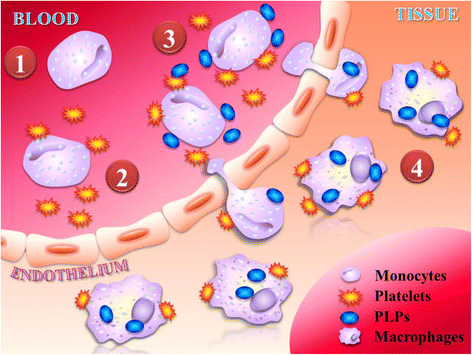
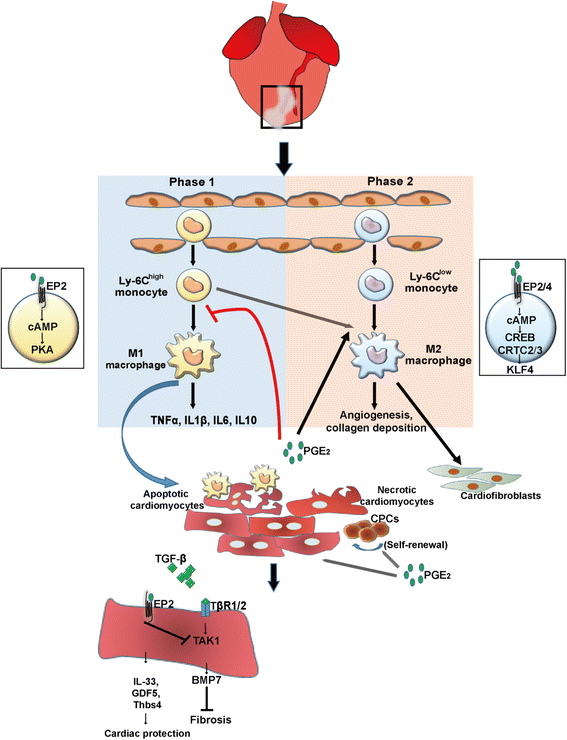
Cardiac inflammation is considered by many as the main driving force in prolonging the pathological condition in the heart after myocardial infarction. Immediately after cardiac ischemic injury, neutrophils are the first innate immune cells recruited to the ischemic myocardium within the first 24 h. Once they have infiltrated the injured myocardium, neutrophils would then secret proteases that promote cardiac remodeling and chemokines that enhance the recruitment of monocytes from the spleen, in which the recruitment peaks at 72 h after myocardial infarction. Monocytes would transdifferentiate into macrophages after transmigrating into the infarct area. Both neutrophils and monocytes-derived macrophages are known to release proteases and cytokines that are detrimental to the surviving cardiomyocytes. Paradoxically, these inflammatory cells also play critical roles in repairing the injured myocardium. Depletion of either neutrophils or monocytes do not improve overall cardiac function after myocardial infarction. Instead, the left ventricular function is further impaired and cardiac fibrosis persists. Moreover, the inflammatory microenvironment created by the infiltrated neutrophils and monocytes-derived macrophages is essential for the recruitment of cardiac progenitor cells. Recent studies also suggest that treatment with anti-inflammatory drugs may cause cardiac dysfunction after injury. Indeed, clinical studies have shown that traditional ant-inflammatory strategies are ineffective to improve cardiac function after infarction. Thus, the focus should be on how to harness these inflammatory events to either improve the efficacy of the delivered drugs or to favor the recruitment of cardiac progenitor cells.
Keywords: Heart regeneration; Inflammation; Macrophage.
Figures
Similar articles
-
S100A9 Links Inflammation and Repair in Myocardial Infarction.Circ Res. 2020 Aug 14;127(5):664-676. doi: 10.1161/CIRCRESAHA.120.315865. Epub 2020 May 21. Circ Res. 2020. PMID: 32434457
-
A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair.Signal Transduct Target Ther. 2021 Feb 22;6(1):79. doi: 10.1038/s41392-020-00455-6. Signal Transduct Target Ther. 2021. PMID: 33612829 Free PMC article. Review.
-
Role of Neutrophils in Cardiac Injury and Repair Following Myocardial Infarction.Cells. 2021 Jul 2;10(7):1676. doi: 10.3390/cells10071676. Cells. 2021. PMID: 34359844 Free PMC article. Review.
-
The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction.Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2206-13. doi: 10.1161/ATVBAHA.112.254409. Epub 2012 Jul 12. Arterioscler Thromb Vasc Biol. 2012. PMID: 22796582
-
Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts.Cardiovasc Res. 2021 Jan 1;117(1):292-307. doi: 10.1093/cvr/cvaa028. Cardiovasc Res. 2021. PMID: 32049348
Cited by
-
Emerging Roles for Immune Cells and MicroRNAs in Modulating the Response to Cardiac Injury.J Cardiovasc Dev Dis. 2019 Jan 15;6(1):5. doi: 10.3390/jcdd6010005. J Cardiovasc Dev Dis. 2019. PMID: 30650599 Free PMC article. Review.
-
U-Shaped Association Between Monocyte-Lymphocyte Ratio and Risk of Cardiac Conduction Block.J Inflamm Res. 2023 Nov 18;16:5393-5402. doi: 10.2147/JIR.S438722. eCollection 2023. J Inflamm Res. 2023. PMID: 38026237 Free PMC article.
-
FcγRIIb Exacerbates LPS-Induced Neuroinflammation by Binding with the Bridging Protein DAP12 and Promoting the Activation of PI3K/AKT Signaling Pathway in Microglia.J Inflamm Res. 2024 Jan 4;17:41-57. doi: 10.2147/JIR.S428093. eCollection 2024. J Inflamm Res. 2024. PMID: 38193040 Free PMC article.
-
Expression of adhesion molecules on granulocytes and monocytes following myocardial infarction in rats drinking white wine.PLoS One. 2018 May 10;13(5):e0196842. doi: 10.1371/journal.pone.0196842. eCollection 2018. PLoS One. 2018. PMID: 29746525 Free PMC article.
-
Kynurenine promotes neonatal heart regeneration by stimulating cardiomyocyte proliferation and cardiac angiogenesis.Nat Commun. 2022 Oct 26;13(1):6371. doi: 10.1038/s41467-022-33734-7. Nat Commun. 2022. Retraction in: Nat Commun. 2025 Mar 3;16(1):2117. doi: 10.1038/s41467-025-57248-0. PMID: 36289221 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

