Biochemical mechanism underlying hypertriglyceridemia and hepatic steatosis/hepatomegaly induced by acute schisandrin B treatment in mice
- PMID: 28086886
- PMCID: PMC5237187
- DOI: 10.1186/s12944-017-0406-9
Biochemical mechanism underlying hypertriglyceridemia and hepatic steatosis/hepatomegaly induced by acute schisandrin B treatment in mice
Abstract
Background: It has been demonstrated that acute oral administration of schisandrin B (Sch B), an active dibenzocyclooctadiene isolated from Schisandrae Fructus (a commonly used traditional Chinese herb), increased serum and hepatic triglyceride (TG) levels and hepatic mass in mice. The present study aimed to investigate the biochemical mechanism underlying the Sch B-induced hypertriglyceridemia, hepatic steatosis and hepatomegaly.
Methods: Male ICR mice were given a single oral dose of Sch B (0.25-2 g/kg). Sch B-induced changes in serum levels of biomarkers, such as TG, total cholesterol (TC), apolipoprotein B48 (ApoB 48), very-low-density lipoprotein (VLDL), non-esterified fatty acid (NEFA) and hepatic growth factor (HGF), as well as hepatic lipids and mass, epididymal adipose tissue (EAT) and adipocyte size, and histological changes of the liver and EAT were examined over a period of 12-120 h after Sch B treatment.
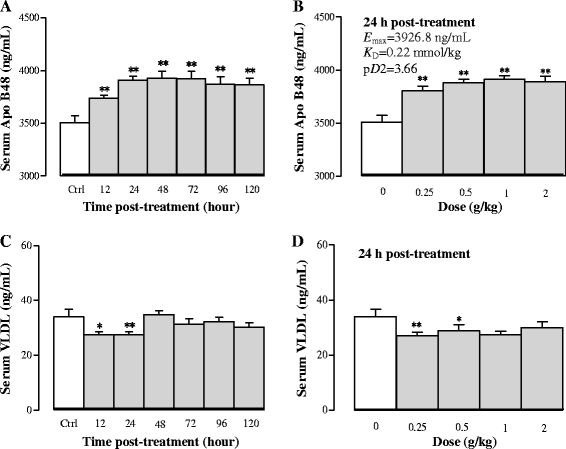
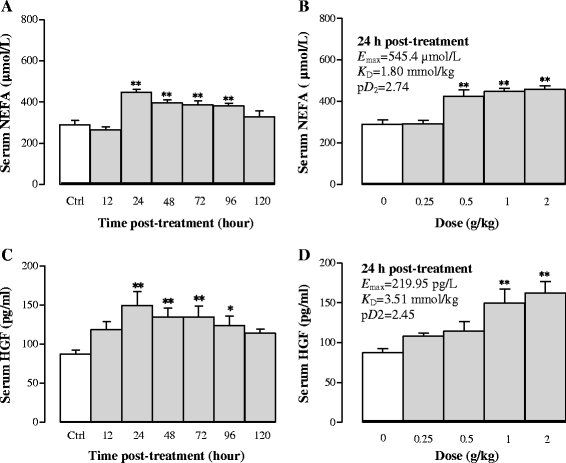
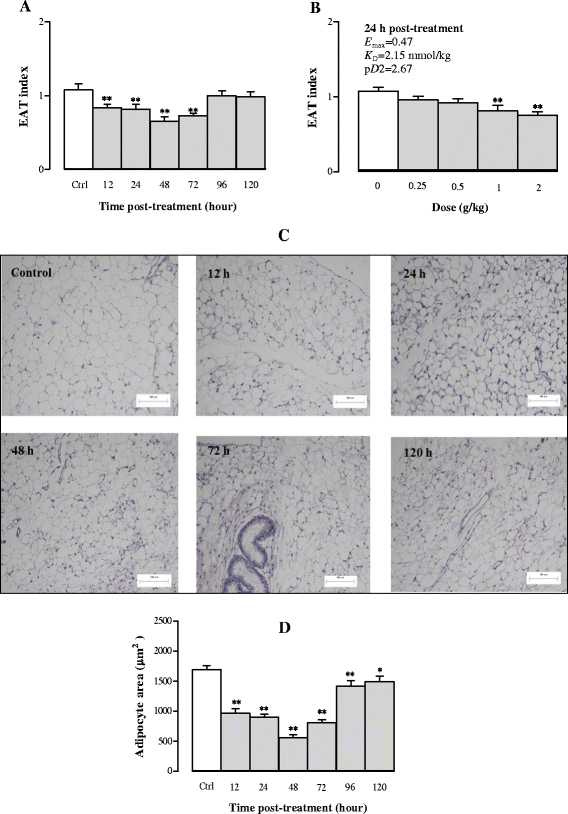
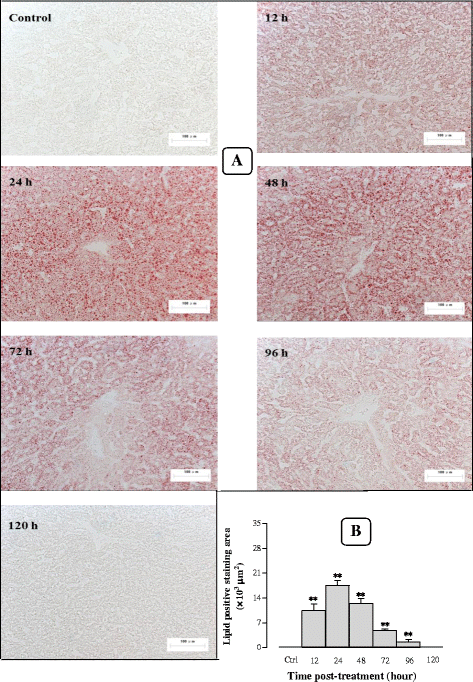
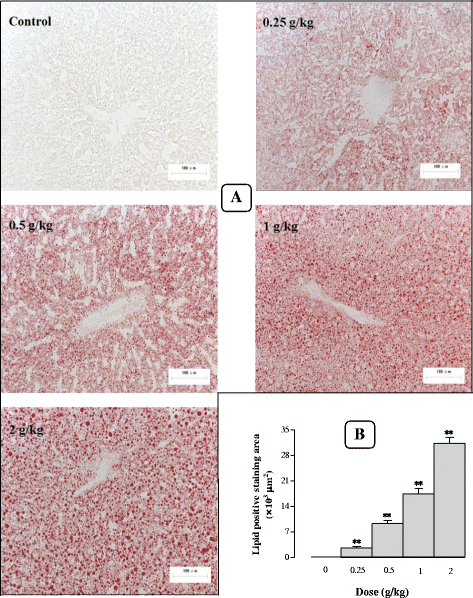
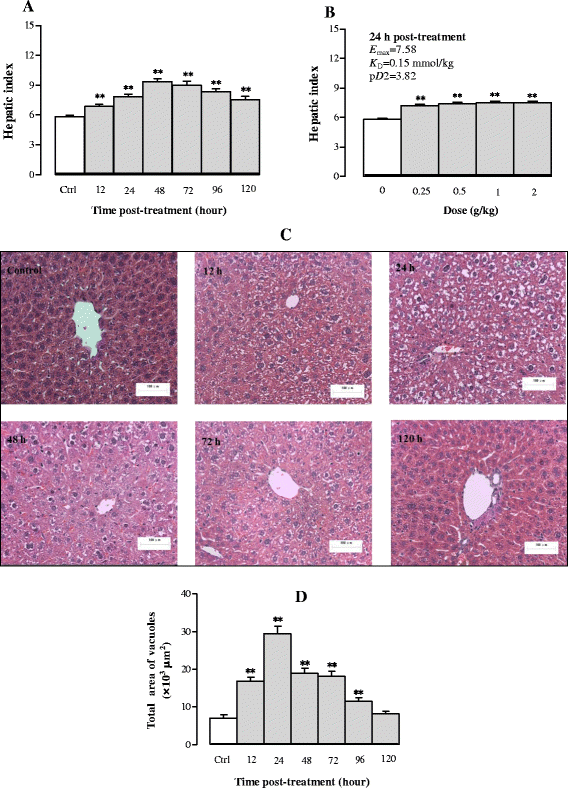
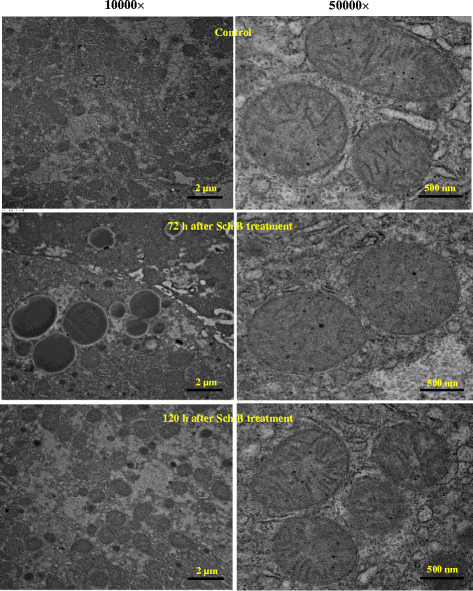
Results: Serum and hepatic TG levels were increased by 1.0-4.3 fold and 40-158% at 12-72 h and 12-96 h, respectively, after Sch B administration. Sch B treatment elevated serum ApoB 48 level (up to 12%), a marker of exogenous TG, but not VLDL, as compared with the vehicle treatment. Treatment with Sch B caused a time-/dose-dependent reduction in EAT index (up to 39%) and adipocyte size (up to 67%) and elevation in serum NEFA level (up to 55%). Sch B treatment induced hepatic steatosis in a time-/dose-dependent manner, as indicated by increases in total vacuole area (up to 3.2 fold vs. the vehicle control) and lipid positive staining area (up to 17.5 × 103 μm2) in liver tissue. Hepatic index and serum HGF levels were increased by 18-60% and 42-71% at 12-120 h and 24-72 h post-Sch B dosing, respectively. In addition, ultrastructural changes, such as increase in size and disruption of cristae, in hepatic mitochondria were observed in Sch B-treated mice.
Conclusion: Our findings suggest that exogenous sources of TG and the breakdown of fat storage in the body contribute to Sch B-induced hypertriglyceridemia and hepatic steatosis in mice. Hepatomegaly (a probable hepatotoxic action) caused by Sch B may result from the fat accumulation and mitochondrial damage in liver tissue.
Keywords: Chylomicron; Hepatic steatosis; Hepatomegaly; Hypertriglyceridemia; Lipolysis; Schisandrin B.
Figures



References
-
- Carpentier AC. Hypertriglyceridemia associated with abdominal obesity: getting contributing factors into perspective. Arterioscler Thromb Vasc Biol. 2015;35:2076–8. - PubMed
-
- Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim Biophys Acta. 2012;1821:819–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

