Can pegylated interferon improve the outcome of polycythemia vera patients?
- PMID: 28086927
- PMCID: PMC5237341
- DOI: 10.1186/s13045-017-0395-1
Can pegylated interferon improve the outcome of polycythemia vera patients?
Abstract
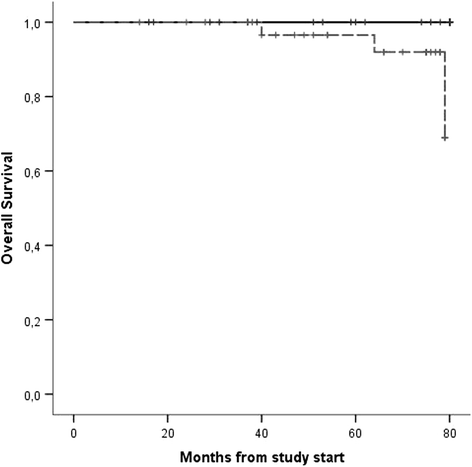
Pegylated interferon (peg-IFN) was proven by phase II trials to be effective in polycythemia vera (PV); however, it is not clear whether it could improve patient outcome compared to hydroxyurea (HU). Here, we present an observational study on 65 PV patients aged 65 years or younger, who received either peg-IFN (30) or HU (35) according to the physician choice. Median follow-up was 75 months. The two cohorts were comparable for patient and disease characteristics. Eighty-seven percent of the patients treated with peg-INF responded, with a CR rate of 70% as compared to 100 and 49% with HU, respectively. Discontinuation rate was similar in the two groups (20% in peg-IFN vs 17% in HU). JAK2 allele burden was monitored in peg-INF arm only, and a reduction was observed in 88% of the patients. No thrombotic events were observed during peg-IFN treatment compared to three on HU. Disease progression to myelofibrosis or acute myeloid leukemia occurred to a patient only in peg-INF, compared to three in HU. Overall, three second malignancies were observed during the study, two in patients who received HU only, and one in a patient largely treated HU who received also peg-IFN for 3 months. Overall survival was significantly better for peg-IFN patients compared to HU, p = 0.027. Our study, albeit limited by small patient and event number and lack of randomization, confirms the efficacy of peg-INF in PV and shows a significant survival advantage for peg-INF-treated patients. Waiting for confirming data from the ongoing phase III trials, our study can support peg-INF as a first-line treatment option for PV, at least for younger patients.
Keywords: Hydroxyurea; JAK2 allele burden; Pegylated interferon; Polycythemia vera.
Figures
References
-
- Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, Bellucci S, Grandchamp B, Chomienne C, Fenaux P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. - DOI - PubMed
-
- Quintás-Cardama A, Abdel-Wahab O, Manshouri T, Kilpivaara O, Cortes J, Roupie AL, Zhang SJ, Harris D, Estrov Z, Kantarjian H, Levine R, Verstovsek S. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon α-2a. Blood. 2013;122(6):893–901. doi: 10.1182/blood-2012-07-442012. - DOI - PMC - PubMed
-
- Barosi G, Birgegard G, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, Kiladjian JJ, Lengfelder E, Mcmullin MF, Passamonti F, Reilly JT, Vannucchi AM, Barbui T. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113:4829–4833. doi: 10.1182/blood-2008-09-176818. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous