Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity
- PMID: 28086928
- PMCID: PMC5234140
- DOI: 10.1186/s12974-016-0777-2
Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity
Abstract
Background: Diet-induced hypothalamic inflammation is an important mechanism leading to dysfunction of neurons involved in controlling body mass. Studies have shown that polyunsaturated fats can reduce hypothalamic inflammation. Here, we evaluated the presence and function of RvD2, a resolvin produced from docosahexaenoic acid, in the hypothalamus of mice.
Methods: Male Swiss mice were fed either chow or a high-fat diet. RvD2 receptor and synthetic enzymes were evaluated by real-time PCR and immunofluorescence. RvD2 was determined by mass spectrometry. Dietary and pharmacological approaches were used to modulate the RvD2 system in the hypothalamus, and metabolic phenotype consequences were determined.
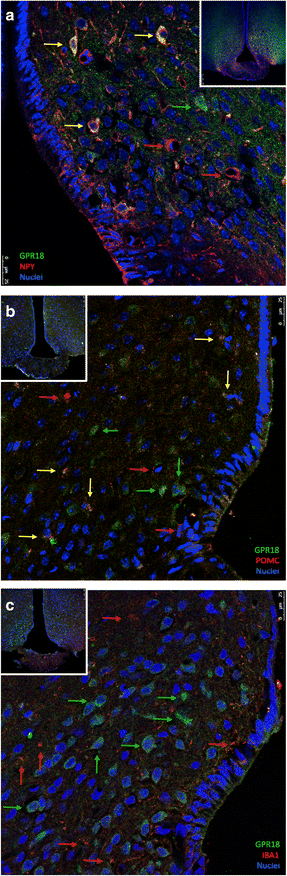
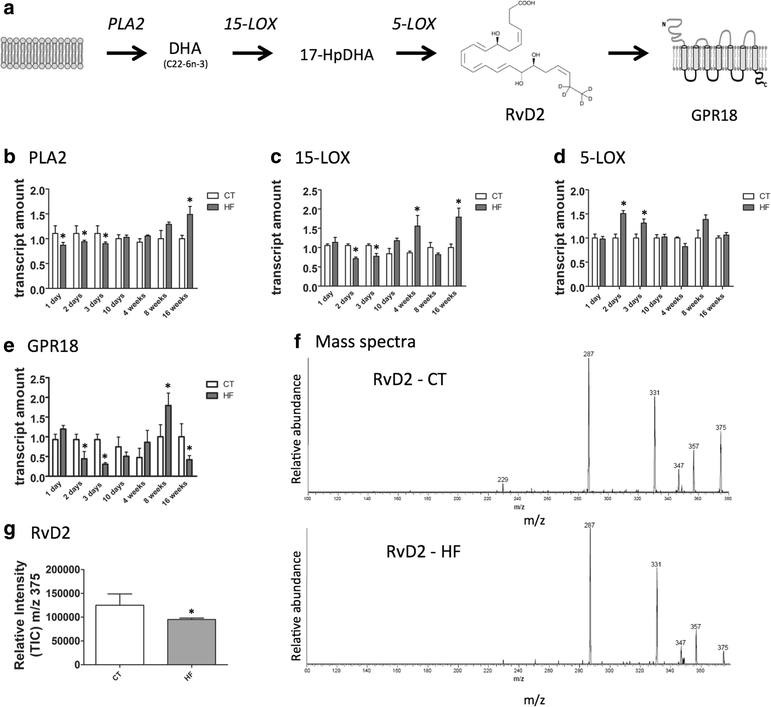
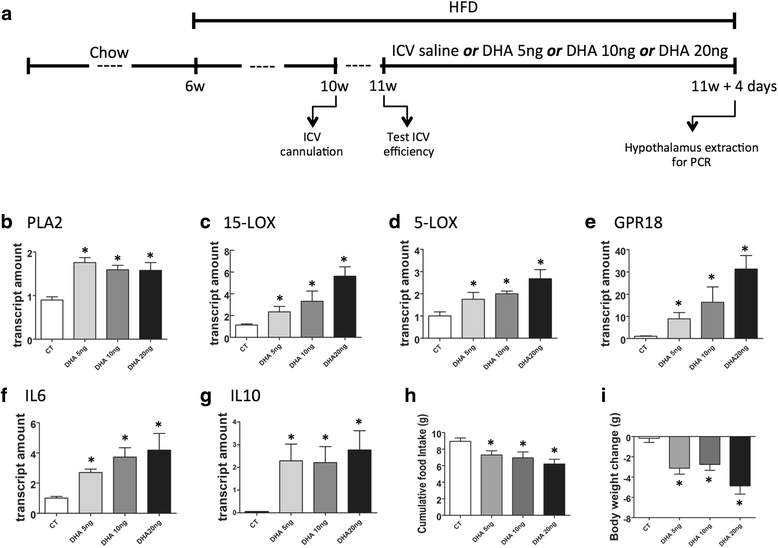
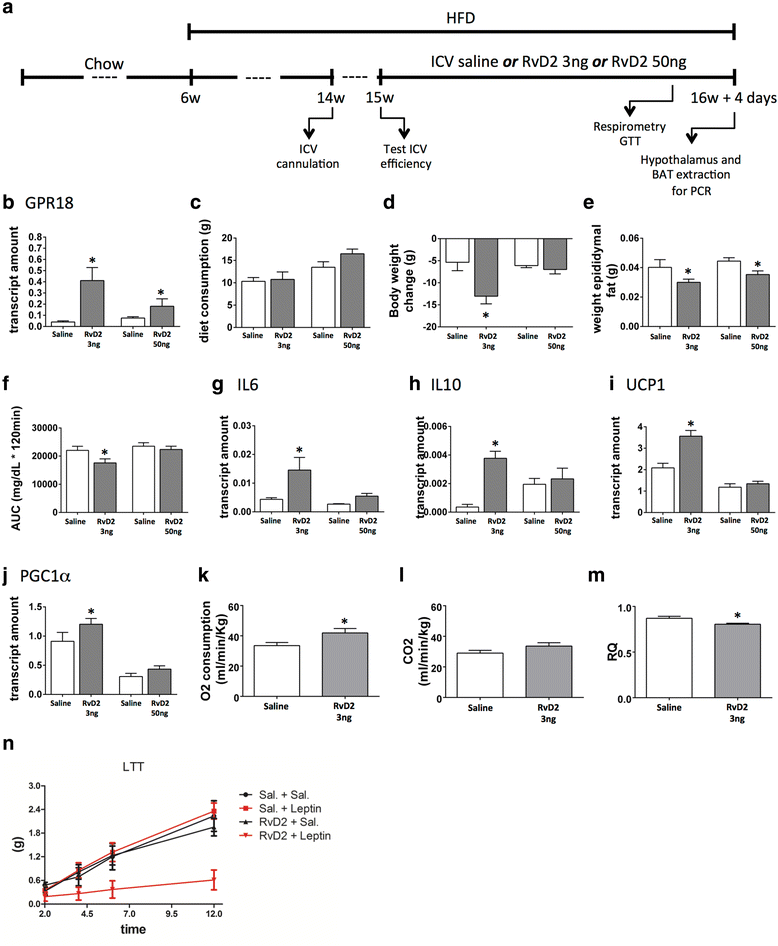
Results: All enzymes involved in the synthesis of RvD2 were detected in the hypothalamus and were modulated in response to the consumption of dietary saturated fats, leading to a reduction of hypothalamic RvD2. GPR18, the receptor for RvD2, which was detected in POMC and NPY neurons, was also modulated by dietary fats. The substitution of saturated by polyunsaturated fats in the diet resulted in increased hypothalamic RvD2, which was accompanied by reduced body mass and improved glucose tolerance. The intracerebroventricular treatment with docosahexaenoic acid resulted in increased expression of the RvD2 synthetic enzymes, increased expression of anti-inflammatory cytokines and improved metabolic phenotype. Finally, intracerebroventricular treatment with RvD2 resulted in reduced adiposity, improved glucose tolerance and increased hypothalamic expression of anti-inflammatory cytokines.
Conclusions: Thus, RvD2 is produced in the hypothalamus, and its receptor and synthetic enzymes are modulated by dietary fats. The improved metabolic outcomes of RvD2 make this substance an attractive approach to treat obesity.
Keywords: Brain; Hypothalamus; Lipid; Metabolism; Nutrient.
Figures

References
-
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. - DOI - PMC - PubMed
-
- Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

